There were an endless debate about whether IQ heritability was spuriously inflated, while the growing evidence, notably from modern techniques (e.g., GCTA), shows a non-negligible heritability in the narrow sense. A large part of the twin method estimates has been validated.
(May 2014. The original article has been improved and extended.)
CONTENT
1. Criticism of heritability
2. Decline of shared environment
3. Range restriction in environment
4. Additive heritability
5. GWAS
6. GWAS: rGE and GxE
7. Twin studies robustness
8. Genetic amplification
9. Generalist genes
10. Causes of rGE and GxE
11. Causes of increasing heritability
12. Problems with rGE theory
13. Biological g
To define it, the heritability is just the proportion of the phenotypic variance in a trait that is attributable to genetic variance, or the genetic variance divided by the total variance of the phenotype. There is broad, and there is narrow heritability. The first one involves additive and non-additive effects, as is the case in the twin methods, notably because of assortative mating causing an artificial rise in additive genetic variance due to children receiving correlated genetic influences from their parents, which means that broad heritability includes some interaction effects. The narrow heritability however is the ratio of additive genetic variance to phenotypic variance, which involves additive genetic effects that are caused by independent effects of alleles, that add up consequently in their effect on a trait, and thus, additivity means that genes and environments act separately. Or, to cite Jensen (1998, pp. 175-176) :
Narrow heritability includes only the additive genetic variance VA, that is, the part of the total genetic variance responsible for the resemblance between parent and offspring. The fixable component of the additive variance is the only part of it that “breeds true” (hence it was referred to by R. A. Fisher as the essential genotype). Therefore, it is only the fixable part of the additive genetic variance that affords the leverage for selective breeding, which can occur either by natural selection or by artificial selection by animal and plant breeders. The nonfixable part of the additive variance results from assortative mating (some degree of genetic correlation between parents on a specific trait). [7] (The coefficient of assortative mating [also called spousal correlation] for IQ in our present population is between +.40 and +.50.)
Broad heritability includes all sources of genetic variance. Besides the additive variance (which is the largest part of broad heritability), there is genotype X environment (GE) interaction: Different genotypes may react differently to the same environmental factor; an environmental condition that is favorable to the-phenotypic development of a certain genotype is less effective or even unfavorable for a different genotype. Also there is genotype-environment (GE) covariance, or the correlation between genetic and environmental factors that affect the development of the phenotype: Genotypes that are more favorable than average for the development of a trait are found with greater-than-chance frequency in environments that are also more favorable than average; likewise for genotypes and environments that are less favorable.
Then there is the nonadditive genetic variance. It results from two types of genetic interactions: (1) genetic dominance (e.g., a dominant and a recessive allele paired at the same locus on the chromosomes might have the same phenotypic effect as two dominant alleles at that locus), and (2) epistasis (a gene at one chromosomal locus affects the phenotypic expression of a gene at some other locus). Dominance and epistatis cause lower correlations between directline relatives (parents-offspring and full siblings) than would be the case if purely additive genetic effects were the only source of genetic variance.
1. Criticism of heritability
Surely enough, twin studies have been overly criticized for having over-estimated heritability (h²). A widely cited study is the Devlin et al. (1997). They evaluate genetic models with and without environmental effects, using 212 studies (or correlations) based on 50,470 distinct pairings. It is the same study as Daniels et al. (1997) that appears in Devlin et al. (1997). What they call shared maternal effects is composed of shared environment (c²) and early environment experienced before separation by adoption (in the case of adopted-out sibling) that they also call prenatal maternal effect. The two types of c² is thus distinguished. Bayesian inference is used to estimate the effect of this early shared environment (not to be confounded with the traditional meaning of c²). Their reasoning reads : “Twins share the womb concurrently, whereas siblings share the womb serially. Hence, although a mother may have similar physiological status and personal habits from one pregnancy to another, the temporal separation between progeny apparently diminishes the correlation of sibling IQ”. They compare models (e.g., III & IV) which attempted to model early common environment to be higher for twins than among siblings with models which constrain maternal effects to be zero (e.g., I & II). Models I & III, unlike models II and IV, assume c² to be equal for twins, siblings and parent-child correlations. Maternal (shared) effect seemed to be essential to achieve the best fit. In the best fit model (III), the total (broad) genetic effect was 0.48 and its components, additive and non-additive were (respectively) 0.34 and 0.15. The maternal environment effects for twins and siblings were 0.20 and 0.05, which figures illustrate the extent of this G-E correlation. The shared environment (c²) estimate was 0.17. According to Lynn (1999), the data was not disaggregated by age categories. Another problem is with the differential early maternal effects. It is known that variance-based estimates distort the real-world effect. The SQRT of these figures give SQRT(0.2)=0.44 and SQRT(0.05)=0.22. It was two times lower, not four times. Furthermore, Bishop et al. (2003, Table 3) did not succeed to replicate Devlin. The likely reason is that Devlin has not considered age effect, at least for maternal environment. Bishop discovered that DZ correlation was indeed superior than nonadoptive sibling correlation at ages 2-4 but the DZ correlation was lower than the nonadoptive sibling correlation at ages 7-10. These numbers indicate a diminishment of special twin shared (environment) effects over time. In any case, Devlin’s indirect evidence for the impact of the so-called “prenatal” maternal effect must be supported by direct evidence, i.e., interventions. But Jensen (1973, ch. 19), Spitz (1986, pp. 155-172) review and Metzen (2012, ch. 5) works do not induce enthusiasm. Contra Devlin, twin-specific prenatal environment does not necessarily lead to heritability over-estimation. It was Lee’s (2010) opinion. And Sesardic (2005, p. 108) put it this way :
It is particularly with respect to prenatal environments that MZ twins are sometimes more different than DZ twins. For example, a substantial proportion of MZ twin-pairs has the third blood circulation system (the so-called “Chronic Fetofetal Transfusion Syndrome”) that leads to serious developmental asymmetries and birth-weight differences, which all tend to increase the MZ environmental variance (cf. Hay 1985: 227). Relying on this kind of empirical evidence, some authors have claimed that the twin research method actually underestimates the true magnitude of heritability, rather than overestimating it (see Price 1950, Munsinger 1977, and references in Bouchard 1998: 270).
And yet, non-additivity remains the central area of discussion. Chipuer et al. (1990, Table 4) modeled IQ components, using Bouchard & McGue (1981) data, with a total N of 59,680 for all pairing groups (MZ, DZ twins, siblings, cousins, etc.). They arrive at a value of 0.32 and 0.19 for additive and non-additive genetic effects, and trying to fix the D component (non-additive genetic) at zero leads to a large deterioration in fit. And the same thing happens when they attempt to set the shared (s) and non-shared (n) environmental parameters to be equal across all groups. That is an indication that the environments are not similar across those groups. The (passive) gene-environment correlations again seem to be evident, with shared environmental parameters of 0.33 for cousins, 0.47 for siblings, and 0.59 for twins. Again, the groups were not disaggregated by age. Besides, Chipuer noted that his estimates may not be extended to the general factor (g) of intelligence. Indeed, it seems possible that g is more heritable than the cognitive dimension (e.g., spatial, speed, verbal, memory factors) of IQ tests or the IQ subtests, based on MCV tests, and perhaps on regressions and CFA-SEM analyses (Cardon et al., 1992; Luo et al., 1994; Rijsdijk et al., 2002; Luciano et al., 2004). These studies have been reviewed elsewhere.
If the studies use observed correlations, measurement errors could have downplayed the impact of genetic effects. Bergen et al. (2007) meta-analyze the existing studies that have given IQ measures at different points in time. Aggregation reduces measurement errors. The regression of IQ and age reveals a predicted h² of about 0.45 and 0.80 for ages 5 and 35. The h² increase per year was 0.012.
Unlike Chipuer (1990), van Leeuwen et al. (2008) did not succeed to reveal the passive GE correlation in their twin study. Two models were compared. One is social homogamy. The other is phenotypic assortment, where the partners choose each other because they live in the same social environment, not because in function of their phenotypes, e.g., traits related to intelligence, but which, in the end, results in the partners having similar phenotypes because people with same intelligence live in the same social environment. In the social homogamy model, GE correlation, or cultural transmission, was present but it has worse fit to the model, that is, less likelihood than phenotypic assortment. In this chosen (best) model, there was no passive rGE. They even stated that “if there is GE correlation, the role of parents seems limited to responding to the needs and interests as indicated by the child”. Incidentally, van Leeuwen et al. (2008, pp. 78, 85-87) report large effect of GxE interaction (due to nonshared environmental influences), -0.30, not in line with Jensen (1973, pp. 173-174), Plomin et al. (1988, pp. 240-249) or Finkel & Pedersen (2001). Negative r must be regarded as low IQ people being more sensitive to environmental effects. Regarding rGE, once again, Rice et al. (1986, 1988, 1989) found evidence of (passive) GE correlation, i.e., cultural transmission to the child IQ through familial environment assessed by HOME variable, but only at ages 1-2. Not after. Plomin et al. (1997) also examine the CAP (1-16 yrs) and found no GE correlations, as expected given the near-zero maternal and/or paternal environmental transmission to the child’s phenotype. Fulker et al. (1988) reach the same conclusion. Cultural transmission was also absent in the CAP for child’s reading performance, at age 7, 12, and 16 years (Wadsworth et al., 2002). In the adult MZA sample of the MISTRA Johnson et al. (2007, Table 9) correlated MZA pair difference in g with MZA pair difference in environments of adoptive homes (method known as cross-twin) but these effects turn out to be small and their implied effect on either IQ or g is zero. Thus, no rGE effects owing to rearing environments.
Jensen (1976) himself has estimated the (passive) GE correlation to be 0.07, with h² and e² being 0.65 and 0.28. Among the set of possible equations involving negative rGE, the average rGE was -0.24 and the average h² was 0.84. Jensen explains that it is possible to conceive a negative rGE. This would happen if, among other things, less able children are susceptible to receive more attention from their parents and professors, and perhaps even their brighter siblings, although Jensen argues that a positive rGE regarding IQ seems more likely than a negative rGE. In general, he says, rGE increases when the difference in MZ and DZ environmental correlation increases, or alternatively if assortative mating is less. Jensen emphasizes the importance of making a distinction between imposed environment, such as passive GE, and chosen environment, such as active GE.
Loehlin & DeFries (1987) argue however that the (passive) GE estimates were too low because they all ignored measurement errors. They estimate the corrected GE at around 0.30 if we accept a test (IQ) reliability of 0.83 for children (0.83 based on Burks (1928) estimates and 0.78 based on Horn et al. (1979) estimates). The uncorrected GE correlation, instead, was around 0.15.
Loehlin (1989, Table 8) attempted to estimate the non-additivity of behavioral traits, including IQ (using Bouchard & McGue 1981 correlations). The equal environment assumption (EEA) can be tested by modeling the equality of c² influences between MZ and DZ. The model fit did not improve when c² is set to be different for MZ and DZ. Thus, the EEA cannot be rejected. Heritability was estimated through direct and indirect equations. Loehlin was motivated by Plomin’s impression that “estimates of the heritability of IQ using direct methods (e.g., from the correlations of identical twins or other relatives reared apart) seem to run higher than those obtained via indirect methods (such as the comparison of identical and fraternal twins, or biological and adoptive siblings).” (p. 1290). The narrow h² estimates was 0.41 and 0.30 for the direct and indirect methods, respectively, while the non-additive genetic effect (d²) was 0.17. Thus, the broad heritability (h²+d²) was 0.58 and 0.47. The sample should have probably been decomposed by age groups. Loehlin’s interpretation of differential h² estimates reads : “It seems unlikely that this is primarily a genetic phenomenon. The genes presumably do not vary their biochemical action as a function of how they are going to be studied some day. From an environmental point of view, however, an interesting thing to notice about the direct and indirect methods is that the direct methods all depend on comparing individuals who have not been reared together in the same families, whereas the indirect methods all involve comparisons among individuals who have been reared together. This suggests that some feature of family interaction may serve to attenuate the effects of the genes in creating resemblance among family members, a possibility obviously susceptible to further investigation.” (p. 1291). Remember. In twin studies, direct h² is based on correlation between MZ twins reared apart (rMZA), indirect h² is obtained by doubling the reared together MZ-DZ difference (2*(rMZT-rDZT)). In adoption studies, direct h² is obtained by doubling the correlation between biological parent and adopted child, and provides crude index of narrow h², and indirect h² is based on the difference between the correlation of adopted children and their adoptive parents and the correlation of natural children reared in the same family with their biological parents. In sibling studies, direct h² is based on the correlation of siblings reared apart, and the indirect h² depends on the difference between correlations of nonadoptive and adoptive siblings reared together.
Now, the question needs to be solved. Plomin & Loehlin (1989) examine the possibilities. Selective placement can increase h² in adopted-apart biological relatives, but this does not explain the differential h² in twin design because studies of MZA don’t usually report selective placement and the MZA are adults, and at this age, c² is supposed to be of little importance. Also, the CAP (adoption) data reveals no such artifact. The GE interaction is not a plausible factor because its effect will increase the difference. Passive GE correlation is not a candidate either, because the acceptance of EEA implies that MZ and DZ receive the same (shared) environments, i.e., equal c², which appears to be true. Also, the absence of selective placement in adoption design would moderate passive GE correlation found among adopted-apart biological relatives. Finally, passive rGE should increase similarity among relatives reared together more than among relatives reared apart, so that in the end, passive rGE would more likely contribute to a reduction in these differences in h² estimates. The reactive/active GE correlation also will not explain this difference because they accentuate the resemblance of adopted-apart biological relatives and reared-together biological relatives but not for adoptive relatives. As a result, the difference in h² will remain. Age, at first glance, could be an important factor because MZA are usually older than MZT subjects, but that cannot explain why direct h² is higher than indirect h² in siblings and parent-offspring designs. The last possibility was the within-family environments (e²) : “Can it be a coincidence that indirect estimates are all based on pairs of individuals who are reared in the same family, whereas direct estimates are all based on pairs of individuals who are not reared in the same family? Within-family environmental factors are important for IQ, especially after childhood (Plomin and Daniels, 1987); however, uniform within-family environmental factors will lower all familial correlations equally and thus leave unchanged indirect heritability estimates derived from differences in correlations.” (p. 339). One obvious, predictable answer is the contrast effect. Among twin pairs, it may emerge either because the parents attempt to accentuate their differences or that the twins themselves seek different paths in order to forge their own identities by, e.g., accentuating the characteristics that differentiate them. But, as they point out, empirical evidence in the field of personality and cognitive ability find no evidence of the contrast effect. Thus, none of these proposed explanation play a major role. Unless the mystery is solved, heritability based on these classical methods can and will be subjected to criticism.
2. Decline of shared environment
Another approach to estimate heritability, initially proposed by Segal (1997, 2000; Segal et al., 2007, 2012; & Hershberger, 2005), that circumvents many of the criticisms is the so-called within-family adoption design study, also named “virtual twins” (VT) study in reference to unrelated siblings of same age, although, in reality, there are two classes of sibship, specifically, the adopted-adopted and biological-adopted pairs. The relationship of the parents with biological and adoptive children mirrors the correlation of MZ and DZ twins. Doubling the difference of these correlations give an estimate of h². The correlation among unrelated same-aged siblings gives a direct estimate of shared environment (c²). Segal (1997) highlights the advantage of the approach as follows : “Less frequently mentioned concerns associated with adoptive sibling studies of intelligence are sibling differences in age at testing, age at placement, number of previous residential arrangements, and early health history. For example, new sources of genetic variation associated with cognitive development at age 7 years have been demonstrated (Fulker, Cherny, & Cardon, 1993), so that differences between adoptive siblings above and below this age might be larger, relative to other pairs. Such measures are sometimes partialed out of the variables of interest, are granted cursory attention, or are not considered at all. Another problematic issue is the differential impact of family or cultural events on siblings who differ in age. Differences in such characteristics could, conceivably, affect adoptive siblings’ similarity in intellectual development. Dumaret and Stewart (1985) noted the difficulties in matching environments between adoptive and biological children for comparative purposes. They documented intellectual and behavioral effects associated with the rearing situations (stable vs. unstable) of siblings who were separately reared in contrasting environments (low socioeconomic status biological family vs. high socioeconomic status adoptive family or foster home/children’s home).” (p. 381).
For example, in Segal (1997), the unrelated same-aged sibling intraclass correlations of 0.17, -0.01, 0.29, for total IQ, VIQ, PIQ, (N pairs =21, mean age of 8 years), were much lower than either biological siblings reared together (0.47) or DZT (0.60) and MZT (0.86) twins or MZA (0.78) twins in other studies (Devlin et al., 1997), thus indicating substantial genetic effects but also non-trivial shared environmental effects. Segal (2000) extends her previous study and uses an enlarged sample (N=90, mean age =8 years) and finds intraclass correlations of 0.26, 0.23, 0.24, for total IQ, VIQ, PIQ, i.e., a non-trivial c² effect. These estimate are close to the correlation of siblings reared apart (0.24). The profile correlation across IQ subtests was 0.08, given 8 subtests, compared to 0.45 and 0.24 for MZ (68) and DZ (35) twins, given 10 subtests, from an earlier twin study of Segal (1985). With this method, passive rGE is revealed if the correlation of the biological-adopted sibling pairs is higher than that of the adopted-adopted pairs, which was the case for total IQ (0.33 vs 0.20) PIQ (0.31 vs 0.16) but not VIQ (0.23 vs 0.20). In the same but extended sample of 34 biological-adoptive pairs and 79 adoptive-adoptive pairs, for 107 families, Segal & Hershberger (2005) estimated a passive rGE of 0.09, 0.15, 0.47, for IQ, VIQ, and PIQ, respectively. The mean age was also 8 years, therefore, the GE and rearing environmental effects haven’t declined yet. Later age at adoption and age entered into family correlate negatively with biological-adoptive pairs. Segal et al. (2007) report in a follow-up sample of 43 pairs that the intraclass correlations at time 1 (age 5) and time 2 (age 10) amount to 0.30 and 0.11 for total IQ, 0.24 and 0.17 for VIQ, 0.27 and 0.06 for PIQ. The decline of c² is made crystal clear. Segal et al. (2012) report results from an extented sample of 142 pairs, the intraclass correlations were 0.28, 0.22, 0.26, for total IQ, VIQ, PIQ, which accords with previous reports. But when they compare biological-adopted and adopted-adopted pair correlations in their study with the correlations reported in earlier studies, Segal data shows serious departure because in other studies there were no meaningful differences in the correlation for the two pairs in total IQ and VIQ. The exception was for PIQ (0.24 vs 0.05) based on Horn et al. (1979). What these authors do not report is that the Horn et al. (1982, Table 7) TAP data shows indeed some evidence of passive rGE at ages 5-7 but not at ages 8+. Petrill & Deater-Deckard (2004) analyzed the N2CAP data (mean age of 10 years for biological child and 7 years for adoptive child, 51 families with one adoptive and one biological child) using within-family approach and reach the same conclusion, with parent-offspring correlation of 0.23 and -0.03 for biological and adoptive siblings. These correlations for adoptive siblings were almost not affected after partialling out age at testing, or age of placement, or number of years in the home (the adoptive lived an average of 5.66 years in the home). The heritability can be obtained by doubling the difference in the correlation. But since one figure was negative, h² is 2*0.23=46%, where SQRT(0.46)=0.68 is the correlation between genotypic and phenotypic values, and c² was 0 and e² is 1-h²=0.54. Correction for range restriction gives correlations of 0.29 and -0.04 or h²=0.58 and c²=0. Thus, the corrected e² is estimated to be 1-h²=0.42. Remember that h²+c²+e² must total 100%. In all of these studies, earlier age at home was associated with higher IQ.
Weak effect of c² on IQ is suggested by Jensen (1973, p. 104) who analyzes the adoption data of Burt (1966) and Newman (1937), derives the within- and between-family effects on the IQ and achievement test of MZT and MZA. Between-family effect was much stronger for achievement test, but not for IQ. Its relevance can be best understood if we recall that IQ test is expected to be less culturally dependent than scholastic test. His conclusion reads : “The fact of much greater within than between environmental effects for IQ strongly suggests that the differences between identical twins in IQ arise largely from prenatal factors rather than from influences in the social-psychological environment. Just the opposite conclusion would pertain in the case of scholastic achievement.” (Jensen, 1970, p. 145). This accords with later works concluding that the effect of c² on IQ is not really important, like in most other behavioral trait (Rowe, 1994). Although Burt’s data is entirely coherent with other data, Jensen (1998, p.198 fn.9) argues that Burt did not manipulate his data but that the errors in Burt’s report of the data makes it justified not to include it in a summary.
3. Range restriction in environment
A known criticism to heritability study comes from Stoolmiller (1998, 1999) who argues that range restriction in environments will under-estimate the effect of rearing environments, especially in adoption design study if restriction in environments is applied to adoptive families. Loehlin & Horn (2000) disagree with Stoolmiller because his model predicts positive skew in the distribution of children’s IQs in adoptive families, whereas in the TAP data, the skewness was negative. They also argue that Stoolmiller’s argument only applies to childhood IQs. The fact remains that the parent-offspring IQ correlation in adoptive families tends toward zero in adulthood. An illustration is given in Loehlin et al. (1989). The path from shared environment (c²) to phenotype at age 3 was 0.25 but at age 14 it was -0.11. Surely, the negative impact of c² on IQ was not expected. Furthermore, Horn et al. (1982, Tables 10, 15, 16) explained that the TAP has double range restriction, i.e., in both biological and adoptive parents, and their opposite effects (environmental vs genetic) tend to cancel out. When they re-analyze the TAP data by splitting the samples into low/high parental IQ and SES, they found that parent-child correlations do not show too serious departure from the total sample, except for SES measures in adoptive families. In any case, h (but also c) of adopted children’s IQ was higher in the lower SES (adoptive family) groups of the TAP. On the other hand, lower IQ in adoptive families is associated with lower h (and higher c) whereas lower IQ biological mother is not associated with lower h (but is associated with higher c) for adopted children. But most importantly is that the adopted children from low-IQ natural mother have 6.7 IQ points lower than those having high-IQ natural mother but they were placed in low-SES homes where the natural children of these same low-SES homes have IQs 1.4 points higher than the natural children living in high-SES homes, just as if (parental) SES had no positive impact on (child) IQ. McGue et al. (2007) studied the SIBS data and found no effect of range restriction of 41% and 18% in parent disinhibitory psychopathology and SES in the adoptive families (compared to non-adoptive) on the adoptive-sibling correlations for delinquency, drug use and IQ. A screening to ensure representativeness suggests that non-paticipants (but initially eligible) do not differ in social characteristics. Unfortunately, the SD of occupational status among adoptive families is somewhat lower than that of the non-adoptive families. McGue et al. (2007) also say that the variation in the scores of HOME environmental measure in the CAP, for example, was 30% less than in the manual, but they explain that such argument would be valid only if HOME was environmentally correlated with IQ in adoptive-sibling correlations. See also Plomin et al. (1988, p. 244). Scarr & Weinberg (1978) adoption study did not find large effect of rearing environments and yet the standard deviation of their SES measures was comparable to the population. The mean and variance (SD) in IQ scores of biological and adoptive parents were similar, and this implies that the over- (under-) estimation of genetic (cultural) transmission appears very unlikely. Similarly, in the MISTRA sample (Johnson et al., 2007), the h² of 4th stratum g is 0.77 and yet the SD of IQ is 14.8, close to the population estimate. And no range restriction in environmental measures for adoptive groups relative to biological groups has been noticed. Also, Benyamin et al. (2005) report the scottish twin cohort data (1932, N=572, 1947, N=517). The IQ heritability was about 0.70, SD of IQ was about 15-16. Given these estimates were drawn from the entire population, the sample was not selected, so that any factors reducing variation (including environment) should be attenuated. While Stoolmiller (1999) agrees that the CAP has the appearance of a very generalizable study, he advances the idea that “Adoption agencies screen out families with obvious parental or sibling pathology and, in the past, required both marital stability and a medical problem that prevented childbearing” (p. 397). While this statement is true, the inclusion of parents having illness and unbalanced health will render any generalizability to the general population almost impossible.
4. Additive heritability
Suggestion about strong additive component in IQ heritability seems plausible. Given the available twin and adoption data, Lee (2010) believes that “the simplest additive model predicts that first-degree relatives should be half as similar as MZ twins, and this prediction does not seem far from the truth”. Hill et al. (2008) note effectively that rMZ>2rDZ is evidence of non-additive genetic effects while rMZ<2rDZ is evidence of shared environmental influence. Given the large data set provided by Haworth et al. (2010) for twins in childhood, adolescence, and young adulthood, we can test the prediction of an additive genetic model, for which the resulting numbers were -0.32, -0.19, -0.14, respectively. Given the adult data provided by Lee (2009), 0.84-(2*0.37)=0.10 and 0.77-(2*0.36)=0.05, we also infer there is no evidence for non-additive genetic impact. Lee (2009) finally makes the following point :
A large genetic variance in the absence of additive genetic variance is a rather peculiar case even in theory. Consider a trait influenced by a single locus with two alleles. In order for the genetic variance to be completely non-additive, there must be equality between the means of the two homozygotes and also the frequencies of the two alleles. In the absence of such special disordinality and symmetry, substantial additivity must be the rule. … Even in the presence of substantial non-additive gene action, population-genetic theory predicts that most of the genetic variance in a polygenic trait should be additive in nature. Because random fluctuations in allele frequency will lead eventually to the loss of one allele, the long-term expected frequency of a mutable, weakly selected DNA variant in a population of small effective size is very near either zero or one. The rarity of one allele at many loci tends to prevent the kind of symmetrical situation leading to non-additive genetic variance. For example, even if a given pair of loci show a strong non-additive interaction, a low frequency of an allele at one locus means that an allelic substitution at the other occurs against a nearly uniform genetic background and thus exerts a predictable effect.
5. GWAS
Today, the genome-wide association methods provide stronger conclusions than old methods. They consist in correlating the similarity across hundreds of thousands of SNPs with phenotypic similarity pair by pair in a large sample of unrelated individuals. Plomin et al. (2012, pp. 90-92) give an illustration with the following picture :
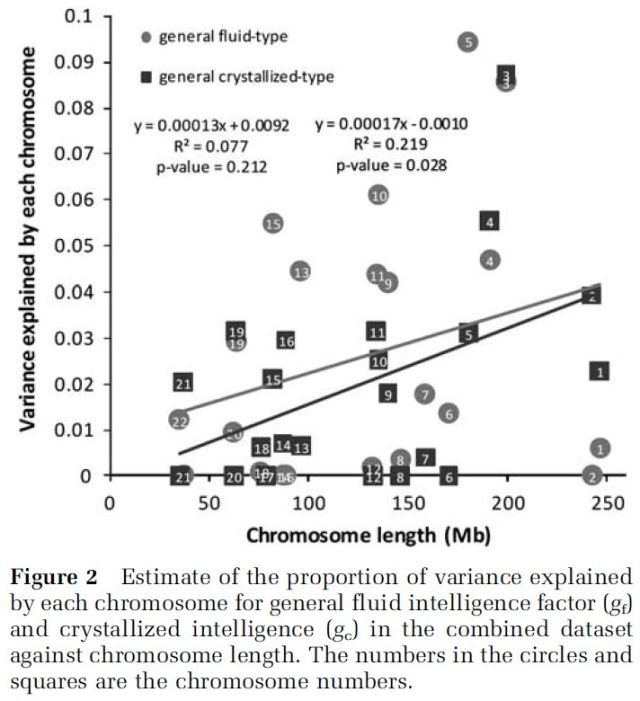
Davies et al. (2011) estimated in a large sample (N=3,511) of unrelated individuals that crystallized (gc) and fluid (gf) intelligence are heritable (40%, and 51% respectively) and polygenic, establishing that the heritability of a complex trait like intelligence is due to a multitude of genes of very small, tiny effects. They report that the proportion of the explained variance for either gc or gf was correlated with the length of the chromosome : “We subsequently partitioned additive genetic variation to individual chromosomes using the software package GCTA, fitting all chromosomes simultaneously, and found that, on average, longer chromosomes explain more variation (Figure 2)”. Their estimates must be considered as being the lower-bound perhaps because their technique (GWAS) that captures the variance in a trait that is due to linkage disequilibrium (LD, which means that certain alleles of each gene (two SNPs) are found/inherited together more often than would be expected by chance) between the genotyped SNPs and the unknown causal variants, i.e., variants having a direct or indirect functional effect on an effect, does not detect all of the genetic variance due to causal genes of low frequency, as Visscher et al. (2010) stated : “If most causal variants for human height have such low frequency in the population that they are not in LD with the (common) SNPs on the commercial SNP arrays then the method we used would not detect much more additional variance than already accounted for by the published genome-wide significant loci”. Visscher et al. (2012) further explicited : “LD is the nonrandom association between alleles at different loci. It is created by evolutionary forces such as mutation, drift, and selection and is broken down by recombination. Generally, loci that are physically close together exhibit stronger LD than loci that are farther apart on a chromosome. The larger the (effective) population size, the weaker the LD for a given distance. … Low LD occurs when the allele frequencies of the unknown causal variants and those at the genotyped SNPs are very different from each other, for example when the allele frequency of causal variants is much lower than that of the SNPs”. In sum, studying rarer DNA variants will lead to larger h² estimates. At the same time, the proposition must fulfill some conditions : “The power of detection for a rare variant is proportional to the product of its frequency (which is small) and the square of its effect size. Hence, rare variants will be detected only if their effect sizes are large enough given their low frequency” (Yang et al., 2011b). Davies et al. study, of course, weakens the Dickens-Flynn model (2001), which postulates that the strong heritability of IQ is explained by the gene-environment correlation (rGE), translating in a positive (or negative) feedback loop. In other words, their model implies non-additive effects.
GWAS is not completely free of potential bias. Population stratification is one of them. GCTA is based upon the assumption that all the individuals sampled in the study descend from a common ancestor. Some G-E confounding can occur because individuals who are more genetically similar also tend to be geographically proximal or belong to the same social class. So, shared environment can be implicated. Population structure or ancestry can be dealt with via PCA (Price et al., 2006, 2010). As Yang et al. (2011b) implied, if the decision to fit the first ten principal components and one chromosome at a time or to fit all chromosomes simultaneously without fitting principal components reveals similar estimates of the variance explained by each chromosome, it suggests that the majority of variance attributed to population structure is well captured by the first ten principal components in these data. The robustness of heritability estimates has been addressed by Davies et al. :
Can the results reported here be explained by population stratification or a correlation between environmental and genetic similarity? A number of reasons suggest strongly that these explanations are unlikely. The results were consistent when we estimated genetic variance within sub-populations and when we adjusted for up to 20 principal components (Supplementary Table 2). The observation that individual cohorts do not show an inflation of the test statistic, but the combined sample does, would require undetected spurious phenotype–genotype associations due to stratification in all cohorts to be in the same direction, which seems very unlikely. We recently showed that when investigating a trait under polygenic inheritance, increasing the sample size would indeed be expected to increase the inflation factor. A correlation between environmental and genetic similarity might occur if similarity due to environmental factors between relatives segregates with the degree of separation. For example, cousins five times removed might be more similar than cousins six times removed because they have a more similar environment. This argument applies to single SNP associations with any complex trait, and there is no evidence that the robustly associated variants from GWAS are spurious in this respect. Moreover, we estimated the actual amount of genome sharing between very distant relatives, which is different from the expected amount of sharing if we knew the entire pedigree of all individuals. In fact, the more distantly related a pair of individuals is from the pedigree, the larger the amount of variation in actual genome-wide sharing around this expectation (see Supplementary Information for further detail). Finally, we partitioned genetic variation to individual chromosomes by fitting the relationship matrices from all autosomes simultaneously in the model. For very distant relatives, as we have in our study, this method is robust to stratification.
What do our results imply about the heritability of intelligence? If our estimated relationships had been based on all causal variants instead of being derived from SNPs that may be in LD with such variants, then we would have had an unbiased estimate of the full narrow-sense heritability. Therefore, our estimates provide a lower bound for the narrow-sense heritability, due to imperfect LD between the genotyped SNPs and unknown causal variants. Our estimates are based upon realized relationships [i.e., the actual amount of genome sharing directly estimated from the SNP data] between very distant relatives and not on pedigree relationships [i.e., the expected relatedness inferred from the family pedigree] between close relatives. This breaks up a possible correlation (confounding) between genetic and environmental factors, since the variation in realized relationships given pedigree relations is large for distant relatives. Our estimates of the phenotypic variance explained by all SNPs are ~0.4–0.5, and we therefore conclude that the narrow-sense heritability for human intelligence is large and consistent with the inference from twin and family studies.
Davies et al. (2011) study has been replicated by Chabris et al. (2012). They applied the same procedure and estimate that about 630 000 SNPs are responsible for 47% of the variance in g, in the Swedish Twin Registry sample. These results corroborate the idea that g is a highly polygenic trait for which common genetic variants have, individually, modest effects. Chabris tried to replicate some earlier studies on the significance of the SNP-g association, with no success, but they concluded that the most likely reason for this was because of the insufficient sample size, and not because of any error in design or execution. That is the inherent problem of a polygenic trait, since very large sample size is required to reach the statistical power needed to detect small effect sizes. Otherwise, the estimates can be quite unstable (see, Trzaskowski et al., 2013d). Nevertheless, according to newscientist, it seems recently that a single gene, HMGA2, has been found to substantially alter IQ.
Plomin et al. (2013), also applied a method called Genome-wide Complex-Trait Analysis (Yang et al., 2011a), GCTA, on a sample (N=3,154) of 12-years old twins from the TEDS survey. Unlike the twin-method estimates that capture both additive and non-additive genetic effects, the GCTA adds up the effect of each SNP. This method only captures additive effects. Non-additive genotypic values within these SNPs (dominance) and between them (epistasis) do not add appreciably to the prediction of the trait. The employed method requires a very large sample, so that the numerous genes associated with cognitive abilities can be detected by using the common SNPs on genotyping arrays. The ratio of GCTA/twin h² is about 0.76 for general cognitive ability (and 0.48 for non-verbal ability). That means GCTA accounts for a great portion of twin heritability. The GCTA and twin heritability are (respectively) 0.35 and 0.46 for general ability, 0.20 and 0.42 for nonverbal, 0.26 and 0.40 for verbal, 0.29 and 0.39 for language ability.
… GCTA provides a lower-limit estimate of heritability because it misses genetic influence due to causal variants that are not highly correlated with the common SNPs on genotyping arrays.
… As mentioned earlier, one possible explanation of the missing heritability is that rare genetic variants have not been considered in addition to the common SNPs that are detected by available DNA arrays.
These results suggest that research using current DNA arrays with their common SNPs could identify genes that account for about two thirds of the heritability of cognitive abilities simply by including larger samples. But why is the cup only two-thirds full? Accounting for the rest of the missing heritability is likely to require other DNA variants not well tagged by the common SNPs on current DNA arrays (Gibson, 2012). Although such data are not currently available, this situation will eventually be resolved by whole-genome sequencing data (Plomin, 2012). Until then, researchers need to consider the possibility that twin heritability estimates are inflated. One argument against this possibility is that twin-based heritability estimates for cognitive abilities are in line with estimates from adoption studies and family studies, even though the adoption and family designs have different assumptions than the twin design does (Plomin et al., 2013).
6. GWAS: rGE and GxE
The first impression that GWAS h² is lower in childhood receives no further support from Benyamin et al. (2014). The IQ h² was 0.46 for ALSPAC (N=5517, age=9), 0.22 for TEDS (N=2794, age=12), 0.40 for UMN (N=1736, age=14). Only the TEDS shows a markedly lower h² as compared with studies among adults. Their supplementary note reveals that both the TEDS and UMN use only 2 verbal and nonverbal tests, whereas ALSPAC gives 10 subtests of Wechsler although it was a short version consisting of alternate items only. The weak/modest increase in h² excludes any possible rGE effect, which theory must foresee h² increase from childhood to adulthood.
Although techniques exist that rules out the impact of population stratification (Visscher et al., 2010; Yang et al., 2011b), it is possible that some artifacts had caused GWAS heritabilities to be under-estimated. Eichler et al. (2010) and Luciano et al. (2013, p. 650) focuse on the possibility that population stratification (heterogeneity) reduces the correlation. On the other hand, Eichler et al. (2010) and Manolio et al. (2009) argue that unmodeled GxE interaction can account for the missing heritability. Epistasis, the interaction between several genes, also known GxG interaction, is another portion of the problem. Eichler et al. (2010) believe that “a significant proportion of the missing heritability is not due to single common variants, nor single rare variants, but rather to rare combinations of common variants”. Similarly, Maher (2008) argues that “Two genes may each add a centimetre to height on their own, for example, but together they could add five”. GWAS do not attempt to model such interaction. On the other hand, Zuk et al. (2012) argue that such gene interactions can inflate the narrow-sense h² if we study the correlations among close relatives. Hopefully, such problem is not present in the study of unrelated individuals. Another but related problem is with the recent method Visscher et al. (2006) had used to determine the heritability of height. Siblings share 50% of their genes on average, with a standard deviation close to 4%, and a range between 37% and 62%. Thus, it is expected that any phenotypic differences between full siblings are entirely due to the effect of genes. The method is appealing, but not perfect. Zuk et al. (2012) believe it suffers from confounds due to genetic interactions because it studies close relatives. Indeed, the affected relatives (in disease study) are more likely to share two nearby epistatic loci in LD that would be unlinked (not LD) in unrelated individuals. Zaitlen & Kraft (2012) attempt to illustrate this problem.
To understand the importance of GWAS heritability, we must remember about the common claim that h² was upwardly biased due to the so-called genotype-environment correlation. Since geneticists believe that passive and reactive rGE cease to exerts their effect in adulthood whereas active rGE strengthen over the development, the most common threat to h² was the plausible confounding due to active rGE. And yet, Scarr & McCartney (1983) expect the active rGE among MZ to be high, among DZ and siblings to be moderate and adopted (i.e., unrelated) to be low. To be clear : “As biological siblings move into the larger world and begin to make active choices, their niches remain moderately correlated because their genotypes remain moderately correlated”. Given that GWAS sample only the unrelated individuals, any plausible active rGE must be either small or non-existent.
7. Twin studies robustness
In sum, GWAS estimates tend to corroborate twin studies that have been under attack in the past with invoked arguments like the implausible equal environment assumption (EEA). But Rowe (1994, pp. 45-47) argues that this assumption is empirically tenable (e.g., Scarr & Carter-Saltzman, 1979; Loehlin, 1989). Although parents, friends and peers do treat the MZ more alike so that their environment will converge due to external social factors, their IQs are not affected in this way. And, importantly, EEA has no consequences on MZA-based heritability because these twins don’t share their environments. As mentioned earlier, EEA can be statistically evaluated. Derks et al. (2006, p. 405) validated the EEA through biometric model. The procedure consists in equating r(Cv) to r(Cc). In ACE models, Cc is denoted as the influence of C common to all observed phenotypes (i.e., observed variables) and Cv the influence of C that is specific to each variable. EEA will not be tenable if all of these parameters are not equal, or if r(Cc), constrained to 1 in MZ twins and freely estimated in DZ twins, appears to be lower than 1 in DZ twins. They found using Osborne (1980) black and white twin data that spatial IQ does not violate EEA.
The argument that heritability is confounded with common environment has been advanced multiple times. For example, age of separation, reunion in childhood, rearing by a relative similarity in social environment are among the candidates. Bouchard (1983) investigates the question of whether these variables succeed to reduce the correlations of MZ twins reared apart. Apart from the fact that the sample size is very small, the result is not compelling, but not surprising. The expectation that adoption would improve IQ is through pathways related to cognitively stimulating rearing environments, educational opportunity and support. Unless the mere contact with biological relatives causes IQ to go up or down, there is no reason to suppose that social environment (rather than cognitive environment) drives IQ (dis)similarity. Later, Bouchard et al. (1990, Table 4) present the MICTAR adoption data and report outcomes of importance (MZA pairs ~42-48; MZT pairs ~37-40). In their adult sample, MZA correlations seem lower than MZT correlations for total IQ (0.64 vs 0.88) and VIQ (0.69 vs 0.88) but not so for PIQ (0.71 vs 0.79) and Raven Mill-Hill composite (0.78 vs 0.76). If being reared together improves similarity among the twins, then within-pair correlations for MZA should be smaller than for MZT. They are, but modestly. As expected, verbal IQ was much more malleable to shared environment. The expectation that MZT correlation will decline with age has also been documented by McCartney et al. (1990, Table 3). Using age in a moderator (regression) analysis, they found negative correlation between IQ rMZT and mean age (per study).
One criticism of twin studies that may be less often heard is that MZ twins are mostly monochorionic (MC) rather than dichorionic (DC) while all DZ twins are dichorionic. The monozygotic of MC type share the same placenta during gestation thereby allowing the twins to exchange blood and all the biological influencing factors (hormones, etc.) it may contain. So, heritability based on MC-MZ twins rather than DC-DZ twins can upwardly bias the heritability. Assuming of course that this prenatal (environment) effect has an impact on any psychological traits such as IQ. When Jacobs et al. (2001) attempted to test the said hypothesis, it is not fully supported by the data. Firstly, mean scores in subtests were similar for MC-MZ, DC-MZ, and DZ twins. Second, and what is the data of interest, is the variance of within-pair difference (through F-tests) for MC-MZ vs DC-MZ. No subtest shows significant difference, except Arithmetic and Vocabulary. Nonetheless, one default with Jacobs study is the use of Chi-square based stats, known to be inflated (deflated) by larger (smaller) sample size. They had 451 pairs; 175 MC-MZ, 95 DC-MZ, and 181 same-sex DZ twins. The sample is not too small, consequently. Nonetheless, effect size needs to be reported as well, which is, hopefully, what they did. Heritabilities for Arithmetic and Vocabulary were 0.67 and 0.82 for (MC)MZ-DZ comparison but the heritabilities were 0.54 and 0.73 for (DC)MZ-DZ comparison. At first glance, these effects are large and meaningful. But considering that the 10 other subtests don’t have such problem, their impact on the total IQ composite score is suspected to be small or modest. Finally, Jacobs et al. (2001) report previous studies that suggest chorion effect to be vanishing over time. Such conclusion, by the same token, offers only meager or no evidence for Devlin (1997) contention that higher DZ twin correlation, compared to sibling correlation, must be attributed to maternal womb effect.
8. Genetic amplification
There is no doubt that IQ heritability in adulthood is higher than 40% or 50%, approaching 70%. On can argue that the rise in heritability is mostly due to active gene-environment (G-E) correlation, that is, individuals build their own environment on the basis on their genotype. An alternative hypothesis is the genetic amplification, which consists in that same genetic influences at an early age will become increasingly important in later age, as they amplify with development, and thereby, the variance explained by earlier genetic influences has become higher. It is said that the high genetic correlation will persist from childhood to adulthood, even with an increase in heritability. Note that amplification can actually occur even with a drop in heritability (e.g., van Soelen, 2012, p. 3873). DeFries et al. (1987) explains the theory in these terms :
How can genetic stability be so high when genetic variance increases so dramatically from about .20 in early childhood to over .50 in adulthood? For example, if the genetic correlation were 1.0, any increase in heritability from childhood to adulthood would present a paradox. Although the magnitude of the genetic correlation in the present study is not necessarily discrepant with the increase in heritability, it is interesting heuristically to consider the possibility that the effects of genes that cause individual differences early in life become amplified during development. In other words, although genetic variation accounts for a smaller proportion of the observed variance of cognitive ability in infancy and early childhood than in adulthood, the effects of many of the same genes may be manifested increasingly as development proceeds.
How could the effects of the same genes be amplified during cognitive development? Consider the genetic control of neurological development as a hypothetical example. Let us suppose that genetic differences among infants are responsible for differences in the complexity of dendritic spines as they develop during the first few years of life and that information-processing capability is a function of the complexity of dendritic spines. Such structural differences might result in only minor functional differences among infants due to their limited information-processing capability. However, functional differences could be manifested to a greater extent during childhood as more information is processed. In this manner, genetic differences would contribute only negligibly to variance in Bayley MDI scores but would increase in their relative contribution as development proceeds. Although the genetic variance is less during early development, the genetic correlations from infancy and early childhood to adulthood could be substantial because the effects of many of the same genes are being expressed at each stage of development.
In any case, these genetic studies are important, because G-E theories imply that IQ heritability is somewhat inflated. But even so, an active G-E model should be seen as being a genetic variation because in this case, the individual creates his own environment on the basis of his genotype; we talk about self-realization (Rowe, 2003, pp. 79-80; Sesardic, 2005, pp. 93-95). As Rowe (1994, p. 92) and sesardic (2005, pp. 99-105) emphasized, it is the imposed (rearing) environment that declines over time. Lee (2010) goes even further by stating that “If the goal of a heritability estimate is a rough bound on the malleability of the trait, then any difficulty in manipulating the environmental mediator may well justify placing its influence on the genetic side of the ledger” (p. 248). Indeed, when the question of heritability is treated, the idea of malleability is never far away.
Given the genetic amplification, a concept that deserves to be treated is the genetic correlation, which could be interpreted as being the probability that a gene influencing one trait will influence another. Jensen (1980, pp. 193-195, see also Jensen, 2006, p. 128, on pleiotropy) dedicated a paragraph to this :
5. Genetic Correlation. Variables x and y may be correlated because of common or correlated genetic determinants. There are three kinds of genetic correlation that are empirically distinguishable by the methods of quantitative genetics: correlated genes, pleiotropy, and genetic linkage.
Correlated genes, through selection and assortative mating – segregating genes that are involved in two (or more) different traits, may become correlated in the offspring of mated pairs of individuals both of whom carry the genes of one or the other of the traits. For example, there may be no correlation at all between height and number of fingerprint ridges. Each is determined by different genes. But, if, say, tall men mated only with women having a large number of fingerprint ridges, and short men only with women having few ridges, in the next generation there would be a positive genetic correlation between height and fingerprint ridges. Tall men and women would tend to have many ridges and short persons would have few. Breeding could just as well have created a negative correlation or could wipe out a genetic correlation that already exists in the population. A genetic correlation may also coincide with a functional correlation, but it need not. Selective breeding in experimental animal genetics can breed in or breed out correlations among certain traits. In the course of evolution, natural selection has undoubtedly bred in genetic correlations among certain characteristics. Populations with different past selection pressures and different factors affecting assortative mating, and consequently different evolutionary histories, might be expected to show somewhat different intercorrelations among various characteristics, behavioral as well as physical.
Pleiotropy is the phenomenon of a single gene having two or more distinctive phenotypic effects. For example, there is a single recessive gene that causes one form of severe mental retardation (phenylketonuria); this gene also causes light pigmentation of hair and skin, so that the afflicted children are usually more fair complexioned than the other members of the family. Thus, there is a pleiotropic correlation between IQ and complexion within these families.
Genetic linkage causes correlation between traits because the genes for the two traits are located on the same chromosome. (Humans have twenty-three pairs of chromosomes, each one carrying thousands of genes.) The closer together that the genes are located on the same chromosome, the more likely are the chances of their being linked and being passed on together from generation to generation. Simple genetic correlation due to selection can be distinguished from correlation due to linkage by the fact that two traits that are correlated in the population but are not correlated within families are not due to linkage. Linkage shows up as a correlation between traits within families. (In this respect it is like pleiotropy.)
Genetic correlation, the extent to which two measures are influenced by the same genetic factors regardless of their heritabilities, should not be confused with bivariate heritability, the extent to which genetic factors contribute to the phenotypic correlation between measures, and is calculated as the product of SQRT of h² of variables X and Y and the genetic correlation of X and Y, or mathematically, h²X*h²Y*rG. One thing must be said, as well, is that a genetic correlation (rG) of 1.0 simply means that both traits have the same genetic determinants, so that any variation in the changes in intelligence between two points in time would be of purely environmental origin (Deary et al., 2012). An rG of 0.50 between VIQ and PIQ would indicate, for example, that a gene associated with VIQ has 50% chance to be associated with PIQ as well. Less-than-unity correlations mean that genetic influences explain both IQ stability and IQ changes during the development (Bishop et al., 2003, pp. 44-45). Because shared environment explains a modest portion of IQ stability, nonshared environment a substantial portion of IQ instability, while g correlates positively with heritability and negatively with nonshared environment, it is predictable (Beaver et al., 2013, p. 436) that intervention would fail to improve g, as the evidence shows.
9. Generalist genes
All this leads us to the generalist genes hypothesis (Kovas & Plomin, 2006a, 2006b), advanced by Robert Plomin, which consists in that the same genes that influence, say, school achievement, will also account for IQ test scores, or a cognitive area (math) correlated with another cognitive area (verbal) due to the same genetic influences. In this way, the correlation between these two variables is due to genetic effects. The two key concepts to understand the theory are pleiotropy (a single gene affecting several traits) and polygenicity (each trait is affected by a multitude of genes), the latter reinforcing the former. The theory received a considerable support from numerous studies on very large sample size (Plomin & Kovas, 2005; Plomin et al., 2007; Haworth et al., 2009a; Docherty et al., 2010; Davis et al. 2009b; Calvin et al., 2012; Chow et al., 2013). GCTA also succeeds to replicate classical twin analyses. The genetic correlations between g and language, mathematics, reading, height, weight, reveal a great similarity between GCTA and twin estimates (Trzaskowski et al., 2013b).
Plomin & Kovas (2005, Tables 4 & 5) mention that high rG also is prevalent for cognitive disability, as reading and math disability had an rG of 0.53. Within mathematics, ability and disability are also genetically correlated, with rG of 0.88. Within reading, ability and disability show rG between 0.70 and 0.90. Within language, ability and disability show rG between 0.60 and 0.90. Comorbidity, the correlation of one (dis)ability with another (dis)ability, is also evident. Bivariate heritability analyses through “DF extremes” (DeFries-Fulker) method yield large estimates between, say, reading disability and math ability. This indicates that one gene found for a certain disability will have a high chance to be associated with ability in some other cognitive domain. Deficits in processes are not genetically independent. Plomin et al. (2007) write in this matter :
A common reaction to this conclusion about generalist genes is disbelief because it goes against the common observation that specific disabilities exist. That is, some children with reading problems have no problem with mathematics and vice versa. If genes are generalists, why do specific disabilities occur? There are three reasons. First, genes are also specialists – genetic correlations are not 1.0. Second, nonshared environments are largely specialists (Plomin & Kovas, in press-b). Third, there is less specificity than it might seem. Even though reading and mathematics correlate phenotypically 0.65 in TEDS, some children with reading problems have no problems with mathematics and vice versa. However, this so-called double dissociation is to be expected on statistical grounds alone and has no bearing on the extent to which different causal processes affect reading and mathematics. A related issue concerning the acceptance of these findings is that, although genetic correlations between learning abilities are greater than their phenotypic correlations, we cannot see genetic correlations in the population in the way that we can see phenotypic associations and dissociations.
Posthuma (2003) and Deary (2006) propose a review of studies showing genetic correlation between g and brain volume, g and cognitive processing (reaction time, inspection time), and in particular the IQ-IT covariance (Luciano et al., 2005) as evidence that a pleiotropic gene model, unlike causal models, has been proved to be the best fitting model, in a large sample (N=2012), hence validating a genetic g hypothesis but not any of the proposed causal paths, either top-down or bottom-up : “In short, there is no causal relationship between IT and IQ; instead, both processes/abilities are partially dependent on the same underlying cause, which analysis has shown to be genetic”. Luciano et al. (2004) had previously demonstrated the existence of a higher-order genetic g among chronometric tests. g is not only confined to psychometric tests. The strong genetic correlations between different cognitive domains (g factor, math, language, reading), usually higher than 0.50, sometimes approaching 1.0, is suggestive of a genetic g. From Deary et al. (2006, pp. 692-694) we read :
From infancy to adulthood: twins. An analysis of first to sixth grade twins (148 MZ, 135 same sex DZ) from the Western Reserve Twin Project suggested that, ‘abilities may be differentially affected by genetic and environmental variation. However, these differential patterns may be simply reflecting the degree to which specific abilities measure general intelligence’. Using 17 ability measures from the Wechsler Intelligence Scale for Children (WISC) and another test battery, they found that all the tests were influenced by genetic sources common to all tests: in other words, they found a genetic g. They also found some genetic effects that were specific to domains of cognitive functioning such as verbal, spatial, perceptual speed, and memory functions. Correlations between phenotypic g loadings and genetic g loadings were 0.88 and 0.76 for the two mental test batteries.
This was investigated further in a Dutch Twin Study in which 194 pairs took Raven’s Progressive Matrices (a test of nonverbal reasoning with a high g-loading) at age 16.1 years and the WAIS at age 17.6 years. The heritability estimates for Full Scale IQ, Verbal IQ and Performance IQ were 0.82, 0.84, and 0.68, respectively. There were no significant effects of shared environment. There were substantial unique environmental contributions, specific to each subtest. The principal interest from these data is the contribution to each subtest from genetic factors. This followed the hierarchical model of mental abilities and, thus, genetic contributions were divided into contributions shared by all tests, those shared by tests covering the same cognitive domain, and contributions to individual tests (Table 3). A general genetic factor contributed a mean of 30% of the variance to all tests (range 8–53%). Note, for example, that 48% of the variance in Raven scores comes from a genetic factor shared with all of the WAIS tests. There are modest contributions from genetic factors at the level of the cognitive domain and the individual test. The heritability of the individual tests ranges from 27 to 76%, with a mean of 56%. The contribution of unique environment to subtests ranges from 24 to 73% with a mean of 44%. The authors concluded: ‘the factorial structure of the WAIS subtests is determined by individual differences in genetic structure (phenotypic g is strongly related to genetic g)’ (p. 207); ‘The covariation among the WAIS subtests and the covariation between the subtests and the Raven in our data are predominantly influenced by a second-order genetic factor and thus strongly support the notion of a biological basis of g’ (p. 209).
Analyses of a Dutch Twin Study have also addressed the changing genetic contribution with age. Twins (N=209 pairs) were assessed by the RAKIT test battery at ages 5, 7, and 10 years, and on the WISC-R at age 12 years. For Full-scale IQ (general intelligence), the contributions (percent variance) were as follows at ages 5, 7, 10, and 12 years: genetics, 26, 39, 54, 64; shared environment, 50, 30, 25, 21 (for the latter three values, the 95% confidence interval includes zero); and unique environment, 24, 31, 21, 15. This decrease in the shared environmental contribution and increase in genetic influence with age from childhood to adolescence was congruent with previous studies. The best-fitting model showed an additive genetic influence which was a common factor, but with age-specific factor loadings; thus, ‘continuity in cognitive abilities is mainly due to additive genetic factors’ (p. 245). Shared environment contributed to continuity and change in cognition, and unique environment contributed to change in development. …
The increase in importance of genetic effects from infancy to childhood has also been demonstrated in longitudinal analyses of twin data from different research groups. For example, in data from 2824 twins analysed using a genetic longitudinal latent g model, heritability increased from 0.17 for a composite score across ages 2, 3 and 4 years to 0.47 at age 7 years. The same genes appeared to affect IQ across age. The term (genetic) ‘amplification’ has been used to describe this pattern of effects.
From infancy to adulthood: adoption studies. The Colorado adoption project included adopted children and their adoptive parents, and also their biological mothers and some biological fathers, as well as control parents and their children. Parents undertook a 3-h test battery, with cognitive, personality, and other assessments. Children were tested at ages 1, 2, 3, and 4 years in the home. At 7 and 12 years, they were seen in a lab. At 9, 10, and 11 years, they undertook a telephone interview. At age 4 years, the h² for specific cognitive abilities were: verbal=0.12; spatial=0.31; perceptual speed=0.21; and visual memory=0.06. These were not significantly different. The heritability of general mental ability increased over time, with the 1, 2, 3, 4, and 7 year h² estimates of 0.09, 0.14, 0.10, 0.20, and 0.36, respectively. A further report applied a Schmid–Leiman-type hierarchical model to the analysis of the genetic and environmental contributions to verbal, spatial, perceptual speed, and memory domains in the year 7 assessment data. A genetic g factor influenced all four domains, with additional domain-specific genetic influences on verbal, spatial, and memory domains. There were no significant shared environment effects; the nonshared environment effects were principally domain-specific, with a shared effect between spatial and memory domains. By age 12 years, with 175 adoptive families and 209 control families, the h² for ability domains derived from a mixture of WISC and Educational Testing Service tests was as follows: verbal=0.26, spatial=0.35, perceptual speed=0.38, memory=0.53. Genetic correlations between the ability domains ranged from 0.27 to 0.58. A simple model, which assumed that the genetic correlations among the four areas were identical, fitted well. Thus about half of the phenotypic association between the cognitive domains was caused by genetic factors and the authors concluded that, ‘specific cognitive abilities appear to be influenced by a pervasive genetic factor whose contribution to each ability does not differ substantially’ (p. 262). The effects of familial environment transmission were nonsignificant.
A more recent analysis of the Colorado Adoption Project asked, ‘what is the pattern of genetic and environmental influence on the stability of cognitive skills from early childhood through late adolescence’. There were 245 adoptive and matched control families. Children by that stage had taken cognitive tests at age 16 years (the WAIS). Phenotypic stability coefficients were moderate to high from age 2 years onwards. For example, the correlation between ages 7 and 16 years was 0.68, and between 12 and 16 years 0.80. At age 16 years, the mean correlation between adoptive siblings’ intelligence test scores was 0.11, and between control siblings was 0.30. Genetic sources were responsible for stability of general cognitive ability from age 1 years to age 16 years. For nonshared environment, only age-specific effects were required, suggesting that they contribute mainly to age-to-age instability or test-error. The mean of the genetic correlations between all ages from 2 to 16 years was 0.78 (range 0.57–1.0).
The Texas Adoption Project involves about 300 families in Texas who adopted children through a church-related scheme for unwed mothers. Children went to adopted homes within a few days from birth and were adopted permanently. Birth and adoptive parents tended to be middle class. Children took Stanford-Binet or age-appropriate Wechsler tests at around age 7 years, at which time adults, excluding birth fathers, took the Adult Wechsler and/or the Revised Beta test. Children were tested on the adult tests at a 10-year follow-up. The correlations of the Beta test between adopting fathers and mothers and their adopted children (with whom they had spent 17 years on average in the same home) were 0.08 and -0.02, respectively. Correlations between fathers and mothers and their biological children were 0.20 and 0.21, respectively. The correlation between the birth mothers and their adopted-away children was 0.33. Three of the six subscales of the Revised Beta exam had specific genetic contributions beyond a general genetic factor. A later analysis of the Texas Adoption Project examined both parent–offspring and sibling correlations. Sibling correlations were higher for biologically related siblings than for adopted siblings, whose scores correlated near to zero. The estimated additive genetic effect on general intelligence was 0.78, for true scores in the population. The authors concluded that, ‘The major contributor to familial resemblance is the genes. Shared family environment has an appreciable effect on IQ when children are small, but this becomes minor by the time they are late adolescents.’
Deary et al. (2012) also reported a rather strong genetic correlation (0.62) between IQ measured at age 11 and IQ measured at age 65-79 in a large scottish sample (N=1,940). In the VETR sample, Lyons et al. (2009) show a phenotypic correlation for AFQT, a highly g-loaded test, measured at age 20 (N=7,232) and 55 (N=1,237), at about 0.74, for which the genetic correlation was 1.0. The fact that Lyons (2009) found no new genetic influences over the adult development is interesting. In another longitudinal sample, Brant et al. (2009; pp. 401-402) report positive genetic correlations between IQs across ages, between 1, 2, 3, 4, 7, 12, and 16 years, that tend to increase with age, “signifying that the same genetic effects are evident from infancy to late adolescence, but that these influences increase in importance across development”. The etiology of ACE development over time shows a similar pattern for high-IQ people. Haworth et al. (2009) also attempted to verify that the ACE etiology among high-g people is the same as the ACE components in the normal distribution of g. The h² and c² for high g were 0.50 and 0.28 and for the normal distribution h² and c² were 0.55 and 0.21. The sample totalized 11,000 twin pairs in UK, US, Australia and Netherlands.
10. Causes of rGE and GxE
The plausibility of larger h² in high-IQ people has been further confirmed by Brant et al. (2013). Unlike heritability-by-IQ interaction, the research on heritability-by-SES or GxE interactions produce conflicting results. Thus the question remains unresolved. Molenaar et al. (2013) explain that the differences in the GxE interactions (where the environmentality is unmeasured) may be due to differences in the methods, e.g., tests used, their numbers, length, reliability, and domain measured, verbal, nonverbal, others, or full IQ, but also (un)representativeness of samples, notably at the extremes, which will cause data non-normality. A more serious threat is the portion of measurement error included in the non-shared environmental component, and reducing the former will likely converge towards more consistent patterns. With respect to heritability-SES interaction, Bouchard (2013) believes that the conflicting results may be explained by the spontaneous changes in IQ induced by G-E correlations. Indeed, Moffitt et al. (1993) research shows IQ change (among children aged 7-13 in the Dunedin sample) appears to be idiosyncratic in its source, and transient in its course, and not associated with any measured environmental factors. Their interpretation that IQ change can be thrown off its trajectory but conform to recovery curves was that active rGE effect was at work behind the apparent level-seeking phenomena. Any environmental effect acting negatively (positively) on the children so as to produce larger (lower) environmentality in lower (higher) SES, thus resulting in obstacles toward achieving potentiality for high(er) IQ, will be negated by such self-correcting process. Brant et al. (2013) further question the relevance of active rGE in IQ development. They found that the genetic influence during the cognitive development comes both from previous, existing genetic influences and new influences, and also report a lower heritability for high-IQ people, and a higher heritability for lower-IQ people (N=11,000). And here is how they reject the role of active rGE in heritability increases :
The most prominent theory of developmental increases in the heritability of IQ posits that across development, individuals gain more scope to shape their own environments on the basis of their genetic propensities (active gene-environment correlation), which causes an increase in genetic influence over time (Haworth et al., 2010; Plomin, DeFries, & Loehlin, 1977). Our results challenge this explanation, as they show a later increase in heritability for individuals of higher IQ. To explain our results in the context of active gene-environment correlations, one would need to posit, counterintuitively, that higher-IQ individuals seek out environments concordant with their genetic propensities later in development than do lower-IQ individuals.
The plausibility of their hypothesis is supported by the fact that IQ differences certainly reflect differences in mental age (Jensen, 1980, pp. 559-562, 1998, pp. 370-371). Nonetheless, if lower IQ children mature earlier, the active rGE may well explain this pattern. The authors are open to the possibility of the amplification model which is, at present, perhaps the most likely and tenable hypothesis. van Soelen et al. (2011) confirmed the amplification model in a pediatric longitudinal sample, consistent with Hoekstra et al. (2007, p. 112) analysis, in which no clear pattern of GxE interaction has been found and in which they show a correlation between verbal and nonverbal abilities that is entirely explained by genetic effects, with stability in nonverbal ability attributed completely to genetic influences between age 5 and 18 (pp. 105-109). van Soelen emphasize the importance of having similar IQ measurement in longitudinal data. When the test administered is different over time, this can introduce age-specific genetic or common environmental influences, resulting in absence of common influences across ages (i.e., no stability).
11. Causes of increasing heritability
More recently, Briley & Tucker-Drob (2013) meta-analysis demonstrates that new genetic influences (innovation; e.g., novel biological changes such as hormonal changes associated with puberty, or environmental changes such as transition from the home to grade school) are likely to explain the increase in heritability at the very early stage of development, but beginning at age 8, genetic amplification becomes predominant, and the effect of new genetic influences drops to zero; the widening of the shaded area (i.e., standard error) with regard to amplification suggests that this parameter is not estimated with much precision, but the authors maintain that their alternative models show the very same phenomenon but more precisely (Figures S3 & S4).
To the question of increasing heritability with age, Jensen (1973, pp. 79-96) advanced the idea of consolidation (C) factor to explain the increasing h² with age. The C factor bears some similarity with the integration factor as described in Woodley (2011). Gains (G) in intelligence over the development become consolidated gains (CG) when the learning from any experiences had been understood. Otherwise, the (new) learning is not maintained without constant rehearsal of the acquired knowledge. But, for whatever exposure to knowledge that is understood, the G accumulates into the C factor. When IQ measurements are given at each subsequent time in point, the IQ contains more and more consolidated gain over time. With increasing C, due to CG over time, heritability is expected to increase.
The term consolidation as used here does not refer to the consolidation of short-term memory traces into long-term storage, but to the assimilation of experience (i.e., learning) into cognitive structures which organize what has been learned in ways that subsequently permit quick and adequate retrieval and broad transfer of the learning in new relevant situations. Stated in simplest terms, C is the process of understanding what one has learned. … When learning takes place without C acting upon it, it is less retrievable and much less transferable for use in solving problems that are more or less remote from the original learning situation. C is what is generally meant by the term intelligence, but it can be manifested, observed, and measured only through its interaction with experience and learning. There can be learning without intelligence (i.e., without C) but intelligence cannot be manifested without learning.
Jensen’s model can account for why the gains are stronger at earlier ages. At this stage, consolidation is easier but becomes increasingly difficult to keep ahead as children progress further into the complexities of the subject matter. This model can also account for why educational programs don’t produce lasting IQ gains. One would think this is due to the increasing heritability with age. But Jensen himself was more elaborate. He stated that the children did not learned the material. The IQ gains had been sustained for several years perhaps because of constant rehearsal or repetition. This illusion of consolidated gains was made apparent at later years when the IQ tests become more complex, difficult, or less alike to the material learned in the past. The gains fade out because of the absence of any consolidation factor and far transfer effect.
Although human brain, a very dynamic organ, undergoes considerable developmental changes from childhood to adolescence, van Soelen et al. (2011, p. 125) point out that those longitudinal changes in cortical thickness is under genetic influences, partly overlapping genetic influences on IQ. van Soelen et al. (2012, twin pairs =113) demonstrate subsequently the existence of a strong genetic influence in cortical thinning during the development, so that “The same genetic factor operates in language areas (e.g., left inferior frontal (superior of) Broca’s and left parietal (superior of) Wernicke’s area). In these areas amplification of the same genetic factors across ages 9 and 12 was observed. Between these two regions, the genetic factors acting on cortical thinning were completely overlapping and this might suggest that the genetic influences acting on cortical development in these areas are implicated in language. We did not find significant influences of common environmental factors on cortical thickness. This is consistent with other neuroimaging studies in both adults (Peper et al., 2007), and children (Lenroot et al., 2009; Peper et al., 2009; Yoon et al., 2010) of both volumetric and cortical thickness measures.” (p. 3878).
In parallel, Smit et al. (2010, Table 8) found that at the earliest stages in life, common environmental factors can play a certain role in head circumference, but disappear very early in life (4 months). And then, between age 5 and 18, individual differences in head circumference were genetically highly stable, as evidenced by the higher genetic correlations for girls (>0.78) and for boys (>0.85), whereas between age 5 and 7, these correlations were near 100%. Not only brain size and IQ correlate between- and within-family, but brain size correlates with IQ at 0.40 and with g-loadings at 0.63 (Rushton & Ankney, 2009; Jensen, 1998, pp. 139-148), meaning that brain size increases by 1 SD with any 0.40 SD increase in IQ (Rushton, 1997, p. 282). Also noteworthy is that Schmitt et al. (2007b, p. 688) indicate that multivariate genetic analyses established a strong genetic relationship between brain areas; the same authors (Schmitt et al., 2007a; Tables 5 & 8, 127 MZ twins, 36 DZ twins, 158 singletons, mean age, 11 yrs) indeed discovered that the great majority of variation in cerebrum, cerebellum, thalamus and basal ganglia was determined by a unique genetic factor, as shown by the high genetic correlations, “As expected from the previous finding that a single genetic factor dominated inter-structure covariance, the genetic correlations between structures dropped substantially when adjusting for total brain volume (Table 7).” (p. 76), “most of the genetic variance is determined by genes that are shared between the major gross neural subdivisions.” (p. 78). Shared environmental factors play no role. IQ shares a common genetic origin with specific brain areas. This has been demonstrated, through the use of cross-trait cross-twin correlation method, by correlating the focal GM and WM densities in one twin with the IQ of the co-twin (Hulshoff Pol et al., 2006). Overall, there is a strong support for the ‘genetic g’ hypothesis, and thus a biological g, seems to be warranted (Plomin, 2003), which weakens the claims made that g is a mere statistical entity.
Davis et al. (2009a), included in the Briley & Tucker-Drob (2013) meta-analysis, show that the genetic and shared environmental influences affecting g from early to middle childhood are quite similar, as illustrated by the genetic and shared environmental correlations, of about 0.57 and 0.65 respectively (N=8,791). Deary (2006) reported a similar result from a large longitudinal study. Their explanation for genetic innovation reads : “In the same way, changes in the developmental neurobiology of the brain may give us some insight into how patterns of gene expression change. For example, during early childhood, cortical thickness and gray matter volumes increase (Shaw et al., 2006). This is accompanied by dendritic spine growth and the formation of new connections in response to the environment. In contrast, middle childhood coincides with the thinning of gray matter in a back-to-front wave over the cortex as it matures by pruning connections (Gogtay et al., 2004), a vital step in the formation of meaningful and efficient neural networks. It is likely that a different set of genes is active during this second developmental period, bringing into play a new range of genetic variants affecting cognition.” (p. 1307).
Trzaskowski et al. (2013a) used an advanced technique, GCTA as a tool allowing to estimate the genetic variance due to linkage disequilibrium between unknown causal variants and genotyped SNPs, which aims to correlate the genomic similarity across hundreds of thousands of single nucleotide polymorphisms (SNPs) with phenotypic similarity in a large group of unrelated individuals (N=2875), children aged 7 through 12. What they discover is a genetic correlation of 0.73 for g. For the sample of the 6702 pairs of twins, it was 0.75. In conclusion, they note : “As noted above, our GCTA estimates of genetic influence account for 74–94% of our twin-study heritability estimates, which implies that most of the missing heritability can be found with additive effects of common SNPs.” (p. 4). The same genes were responsible for genetic influences on g at age 7 and 12, although heritability increased. The established IQ heritability from GCTA studies is interesting in itself when compared to the possible absence of genetic influence for childhood behavior problems from DNA analysis (Trzaskowski, 2013c, pp. 1053-1055).
12. Problems with rGE theory
Numerous problems with G-E correlational theories, commonly used to dismiss IQ heritability, are yet to be underlined. Here is how Neven Sesardic (2005, pp. 113-114) attacks the G-E theory :
This kind of scenario involves four causal factors: G (genetic difference) → C (characteristic that is not directly trait-relevant) → E (environmental influence) → P (trait difference). Whether something like this really occurs can be tested in different ways.
(i) The scenario implies that C will be correlated with P: genetically related individuals are claimed to be more alike with respect to both C and P. One way, then, to sort out the causal connections is to check whether the correlation between C and P will still exist when G is controlled for. If the correlation disappears, this would strongly suggest that G is actually the common cause of C and P, and that G is not causing P via C. The four-link scenario would be undermined.
Essentially this is what Sandra Scarr did when she tested a very popular environmentalist hypothesis (H) in which the externally perceived identity of appearance of MZ twins plays the role of C. According to H, MZ twins are more similar with respect to some phenotypic trait(s) P merely because their genetic relatedness (G) makes them look similar (C), which in turn makes others treat them similarly (E), and this similar treatment by others (E) is the crucial causal influence on P. Scarr (1968) and Scarr and Carter-Saltzman (1982) compared MZ twins (who are 100 percent G-similar and strongly C-similar) with those DZ twins who, being almost indistinguishable from one another, are incorrectly classified as MZ twins (who are strongly C-similar despite being only 50 percent G-similar). Obviously, H predicts that these two kinds of twins should exhibit an increased degree of twin-to-twin similarity with respect to P, because they share the same degree of C (the similar external appearance), the hypothesized “main cause.” But although many phenotypic traits were explored, the prediction was disconfirmed: despite their increased perceived similarity, DZ twins incorrectly classified as MZ were far less similar phenotypically than real MZ twins.
(ii) An alternative testing method would be to check whether another prediction of the above four-link scenario holds, namely whether P-differences within twin pairs are systematically related to their E-differences. For example, if it is twins’ being dressed alike that makes them phenotypically similar, it would follow that those twins who are not dressed alike should show less trait similarity than those who are. John Loehlin and Robert Nichols (Loehlin & Nichols 1976) conducted such a study with a large sample, and the result was essentially negative. They collected information about twins’ different treatments (as reported by their mothers), but the average correlation between a composite measure of these various possible E-factors and a number of psychological traits was very close to zero (0.056).
(iii) Yet another approach is based on a simple idea (Loehlin 1992: 109; Neale & Cardon 1992: 223): take a number of pairs of MZ twins reared apart, and then just check whether there is a correlation between a given environmental measure of one twin and the other twin’s phenotype. If there is no correlation, this would indicate that the MZA phenotypic similarity is not mediated through this particular environmental measure. (In active and reactive G–E correlation the causal relation is G → E → P, and since the twins have the same genotype, G could not influence P via E if there were no cross-correlation between E and P.) Plomin (Plomin et al. 2001: 311–312) used a similar research design in the context of the Colorado Adoption Project but with parent–child pairs instead of twins. Only meager evidence was found for reactive or active G–E covariance: just a few and fairly low correlations were found between the biological mother’s personality traits and the adopted children’s environmental measures.
(iv) If it is the environments shared by MZ twins that cause their phenotypic similarity, one would expect that their similarity would decrease with earlier age of separation, lower degree of contact, and less time spent together. But the massive study of Swedish twins found no effects of this kind (Lykken 1995: 78).
(v) Finally, the most effective way to empirically investigate the issue of G–E covariance is the multivariate genetic analysis. Here, the basic strategy is to look simultaneously at the impact of genetic differences on two variables (environment and phenotype) in the attempt to estimate whether the two effects overlap (and if yes, to what extent). Explorations with this method have not unearthed strong G–E correlations of the kind envisaged by Block and others, and certainly nothing of the order that could put into serious doubt the accepted high heritability estimates for IQ and many other psychological traits.
Precisely, the different types of rGE can be tested by 3 distinct methods (Plomin et al., 2012, pp. 112-117). The passive rGE is modeled by correlating the adoptive parents IQ to an environmental measure (such as HOME variable) and to the children’s IQ. If all those correlations are positive, environmental effect through familial environment is evident. But if the correlation between biological parents IQ with both the HOME and the children IQ is greater than for the adoptive parents, genetic influence (mediation) of the environmental measure is evident. The reactive as well as active rGE can be evaluated by correlating the birth parent’s traits (e.g., IQ) with the environment of their children adopted away. The traits of birth parents can be and is used as an index of adopted children’s genotype, and this index can be correlated with any measure of the adopted children’s environment. Such correlation reveals that the environmental measure reflects genetically influenced characteristics of the adopted children. That is, adopted children’s genetic propensities evoke reactions from adoptive parents. In the case of active rGE, if biological parental IQ is an index of the (adopted-away) child’s genotype, its positive correlation with HOME and child IQ means that the children’s genotype predisposes them to actively seek their own, preferred environment, which in turn is correlated with their IQ. On the CAP data, however, Plomin et al. (1988, pp. 264-269) could not validate reactive or active rGE effects to the extent that biological mother’s IQ was not correlated with HOME or FES variable in the adoptive families of their children. Finally, multivariate genetic analysis can detect every kind of rGE. It would estimate the extent to which genetic effects on one measure overlap with genetic effects on another measure. In this case, genotype-environment correlation is implied if genetic effects on an environmental measure overlap with genetic effects on a trait measure (e.g., IQ). The environmental variable cannot be parental SES because it is uniform to the family, not specific to the child, such as HOME variable.
Another way to test the plausibility of GE correlations is by examining the longitudinal outcome of educational programs aimed to improve IQ and achievement. The picture is that IQ has not been sustained over time whereas achievement has improved at the same time the IQ gains were fading out. To explain this pattern in terms of active GE, which was Dickens & Flynn (2001) take, one needs to argue that the experimental groups, despite being more privileged in terms of social environments, have regressed on their IQ to the level of the control groups because they were seeking environments of poorer quality than what would be expected given their higher academic success compared to the control groups. Such impossibility, by the same token, it also proves that g is not an emergent factor owing to some combinations and interactions of external influences.
13. Biological g
That g is not confined to the psychometric realm must be understood. It is evident that g shows numerous biological correlates (Jensen, 1998; Jung & Haier, 2007; Peper et al., 2007; Schmitt et al., 2007b; Deary et al., 2010; Goh et al., 2011, p. 310; Menary et al., 2013, p. 603; Vuoksimaa et al., 2014), neural mechanisms such as glucose metabolism, biochemical activity, cortical development, and contradicts the ‘anti g-theorists’ who tend to build g from the outside, reducing it to a simple mathematical abstraction. Explanations of these biological correlates have been proposed. For instance, the fact that glucose consumption correlates negatively with IQ would strongly support the idea that more intelligent individuals use their neurons more efficiently. A correlation between callosal thickness and IQ is an indication that additional or better-myelinated callosal pathways can facilitate a more efficient inter-hemispheric information transfer, which would benefit the integration and processing of information. Likewise, individuals with more gray matter (neurons, synapses, and dendrites) and more white matter (myelinated axons) in these areas usually display higher IQ scores. For a review of the studies, see Luders et al. (2009) and Neubauer & Fink (2009). Besides, evidence points out that some common genetic variants are associated with infant head circumference (Taal, 2012).
The existence of additive genetic effects between brain volumes and IQ is also suspected. van Leeuwen et al. (2009, twin pairs =112), by fitting a series of nested models in which the means and variances between MZ and DZ twins were equated, have tested several assumptions (e.g., equality of means and variances between MZ and DZ twins). They continued constraining parameters until the most parsimonious model with still acceptable fit was established, with the choice for the best fitting model based on likelihood-ratio tests. Given the limited importance of D and C components (Table 4) they limit the analyses on AE model. They found that dropping the A component in the saturated AE model led to a significant deterioration of fit, meaning that additive genetic factors make a significant contribution to the variance and covariance in the three brain measures (TBV, GMV, WMV) and the four intelligence measures (Raven, VC, PO, PS). In their discussion section, they write :
Under the causal hypothesis both genetic and environmental correlations should be significant, whereas a significant genetic correlation in the absence of an environmental correlation falsifies the hypothesized causal effect of intelligence. However, when traits are highly heritable (in the range of 90–100%), as is the case in brain volumes, causality (brain volume causes intelligence) cannot be distinguished from pleiotropy (the same set of genes affects brain volume as well as intelligence).
… However, our study shows that only the genetic correlations are significant. In fact 85% to 100% of the covariation between brain volume and intelligence are caused by shared genetic factors. This leaves two options: 1) the relation between brain volume and intelligence is caused by a set of genes which influences variation in brain volume and this variation in turn leads to variation in intelligence 2) pleiotropy: there is a set of genes which influence brain volume as well as intelligence.
But whereas Leeuwen failed to detect a relationship between processing speed (PS) and brain volume, Betjemann et al. (2010) succeeded, in a study showing that the correlation between IQ and brain volume is mostly due to genetic influences. The reason is simply because Leeuwen used only 2 measures of PS while Betjemann used 4 measures. Therefore, Betjemann’s composite score of PS is much more reliable.
In an analysis at the second-order latent g level, Grandy et al. (2013) provide evidence that individual alpha peak frequency, IAF, which had been discussed by Posthuma et al. (2003), correlates with g, and that g mediates the relationship between IAF and some specific ability factors. As they write, “After establishing a substantial correlation between IAF and intelligence at the level of second-order g, we examined whether there was any evidence within our data for specific associations between IAF and first-order factors of perceptual speed, memory, or reasoning. Importantly, estimating residual associations between the IAF latent factor and each of the three first-order latent factors in the presence of the correlation between IAF and g did not indicate any significant correlation between IAF and cognitive abilities beyond the correlation between IAF and g.” (pp. 15-16). Just like g, the authors reported that IAF is not affected by extensive cognitive training. Their association, anyway, supports the theory that differences in intelligence are related to differences in general oscillatory properties in the brain. Although earlier studies reported conflicting result on this matter, Grandy speculates that a possible reason is that the correlation has been computed at the level of individual tests, thus containing a larger portion of error and specific variance. Instead, Grandy uses a g unbiased by measurement errors.
Penke et al. (2010) established that a general factor was not only present among specific cognitive abilities, there is also a general factor of white matter integrity, supporting the views that impaired cortical connection (lowered white matter integrity) is a global process affecting many tracts simultaneously. Eight matter tracts have been factor analyzed, yielding a so-called “common integrity factor” which explains about 45% of the individual differences across all eight tracts. Individual tracts showed no associations beyond what the common integrity factor explained. Besides, a simple RT, 4-choice RT, and inspection time have been factor analyzed, yielding a general information processing-speed factor. This processing speed factor is found to be (modestly) correlated with the common integrity factor (while general intelligence, obtained by factor analyzing mostly the Gf or PIQ subtests of the Wechsler, was not correlated with the integrity factor). When controlling for the integrity factor, the correlations between the speed factor and the eight individual tracts were not significant (excepted one). According to the authors, the absence of correlation between the g-factor and the integrity factor could be explained by the fact that “speed tends to be affected earlier in life by age-related decline … whereas higher abilities are more likely to be maintained by compensatory processes” in the face of age-related cognitive decline.
Subsequently, Penke et al. (2012, Figure 2) conducted SEM analysis to test the possible pathways from three different indicators of white matter tract integrity to general intelligence (g). They discovered that this path was fully mediated by information-processing speed, which is relevant to Jensen’s hypothesis (2006, pp. 207-208, 212, 216-217) of mental speed. They made this assumption since efficient information processing between distal brain regions is thought to rely on the integrity of their interconnecting white matter tracts. Well-connected white matter favors efficient information processing. Jung & Haier (2007) also emphasized on brain functional connectivity to explicate the neural basis of intelligence.
Still related with the causal entity of g in mental abilities, a multivariate SEM study by Shikishima et al. (2009, see p. 258, Fig. 1 & 3) indicates that a common pathway model AE among the other competing models (Table 8), in which a latent factor ‘g’ was posited as a higher-construct (i.e., genetic and environmental factors influencing logical, verbal and spatial abilities through a latent factor), has been proven to be the best fit model to their twin data, confirming g as existing at the etiological level, and thus they were able to represent g as a valid construct.
The common pathway model implies that genetic and environmental effects contribute to a psychological entity, g, per se, rather than directly to each ability, and that each ability is subdominant to g. Alternatively, if g is not an entity but merely an artifact, the model that does not include the latent factor should exhibit a better goodness-of-fit. …
Given the background mentioned thus far, we propose the following two hypotheses. First, the ability that has historically been referred to as the symbol of human intelligence, namely, syllogistic deductive reasoning ability, will be strongly g-loaded. Second, general intelligence g will be identified not only at a phenotypic level but also at its genetic and environmental factor level. …
The better goodness-of-fit for the common pathway model (in which a higher-order construct is hypothesized as an entity) compared with the independent pathway model (in which commonalities are not postulated as an entity) lends plausibility for the existence of g in terms of its genetic and environmental origin.
At first glance, this may not be consistent with van der Maas et al. (2006) mutualism model because they posit that the positive correlations (positive manifold) of cognitive tests, represented by the g factor, are the result of mutually, reciprocal (beneficial) causal interactions amongst distinct elements, during childhood development, that finally give rise to g. They were building g from the outside, making it a hollow concept; g was not a causal entity. According to the model, at the initial phase of the development, there is no positive manifold, cognitive processes being not correlated, meaning that “intelligence, which standard psychometric intelligence tests purport to measure, takes some time to develop in children. Consequently, we expect that it takes some time for the positive manifold, and thus the psychometric g factor, to emerge.” (p. 851). The very fact that Thompson et al. (1985) had detected the presence of genetic g among infants runs against the mutualism model if at this state of age, environmental-cultural effects are not expected to exert their effect yet (McCall, 1981). Instead, it suggests g as a causal entity.
Panizzon et al. (2014) replicated Shikishima (2009) but evaluate 3 different models, first-order g, second-order g, and correlated factors. According to the authors, in the first-order factor model, g is viewed as an independent latent factor that is directly associated with all elementary (measured) cognitive tests and specific cognitive domains are assumed to be uncorrelated with g and with one another, but, in the higher-order factor model, g is a superordinate latent factor that accounts for the correlation among specific cognitive domains, and so, the association of g with elementary cognitive tests is assumed to be fully mediated through the specific domains in the higher-order factor model. The 2nd-order g model constituted the best representation of the data.
One problem may be that none of these authors really demonstrated that g has caused the inter-relationship between the lower-order factors. In any case, Shikishima et al. (2009) remind the reader that the empirical evidence and reality of a biological g does not mean that g itself involves a unique process, as was wrongly believed by some researchers (Hampshire et al. 2012). According to Plomin (2003), “It should be noted that genetic g does not necessarily imply that there is a single fundamental brain process that permeates all other brain processing, such as a ‘speedy brain’, neural plasticity, or the quality and quantity of neurons. It has been proposed that g exists in the brain in the sense that diverse brain processes are genetically correlated. For example, gray and white matter densities in diverse brain regions are highly heritable, substantially intercorrelated across brain regions, and correlated genetically with g”. Jensen (1998, pp. 130-132, 259-261) specifically stated the following :
The g factor, which is needed theoretically to account for the positive correlations between all tests, is necessarily unitary only within the domain of factor analysis. But the brain mechanisms or processes responsible for the fact that individual differences in a variety of abilities are positively correlated, giving rise to g, need not be unitary. … Some modules may be reflected in the primary factors; but there are other modules that do not show up as factors, such as the ability to acquire language, quick recognition memory for human faces, and three-dimensional space perception, because individual differences among normal persons are too slight for these virtually universal abilities to emerge as factors, or sources of variance. This makes them no less real or important. Modules are distinct, innate brain structures that have developed in the course of human evolution. They are especially characterized by the various ways that information or knowledge is represented by the neural activity of the brain. The main modules thus are linguistic (verbal/auditory/lexical/semantic), visuospatial, object recognition, numerical-mathematical, musical, and kinesthetic. …
In contrast, there are persons whose tested general level of ability is within the normal range, yet who, because of a localized brain lesion, show a severe deficiency in some particular ability, such as face recognition, receptive or expressive language dysfunctions (aphasia), or inability to form long-term memories of events. Again, modularity is evidenced by the fact that these functional deficiencies are quite isolated from the person’s total repertoire of abilities. Even in persons with a normally intact brain, a module’s efficiency can be narrowly enhanced through extensive experience and practice in the particular domain served by the module. …
But at some level of analysis of the processes correlated with g it will certainly be found that more than a single process is responsible for g, whether these processes are at the level of the processes measured by elementary cognitive tasks, or at the level of neurophysiological processes, or even at the molecular level of neural activity. If successful performance on every complex mental test involves, let us say, two distinct, uncorrelated processes, A and B (which are distinguishable and measurable at some less complex level than that of the said tests) in addition to any other processes that are specific to each test or common only to certain groups of tests, then in a factor analysis all tests containing A and B will be loaded on a general factor. At this level of analysis, this general factor will forever appear unitary, although it is actually the result of two separate processes, A and B. … However, the fact that g has all the characteristics of a polygenic trait (with a substantial component of nongenetic variance) and is correlated with a number of complexly determined aspects of brain anatomy and physiology, as indicated in Chapter 6, makes it highly probable that g, though unitary at a psychometric level of analysis, is not unitary at a biological level.
To summarize, the additive genetic influences on g is quite high, the G-E correlational model not clear, and the very fact is that g seems really to be a biological entity, and a causal entity as well, meaning that strong inferences can be drawn from it.
References.
- Beaver, K. M., Schwartz, J. A., Connolly, E. J., Nedelec, J. L., Al-Ghamdi, M. S., & Kobeisy, A. N. (2013). The genetic and environmental architecture to the stability of IQ: Results from two independent samples of kinship pairs. Intelligence, 41(5), 428-438.
- Benyamin, B., Wilson, V., Whalley, L. J., Visscher, P. M., & Deary, I. J. (2005). Large, Consistent Estimates of the Heritability of Cognitive Ability in Two Entire Populations of 11-Year-Old Twins from Scottish Mental Surveys of 1932 and 1947. Behavior Genetics, 35, 525–534.
- Benyamin, B., Pourcain, B.St., Davis, O.S., Davies, G., Hansell, N.K., Brion, M.-J.A. et al (2014). Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Molecular Psychiatry, 19, 253–258.
- Bergen, S. E., Gardner, C. O., & Kendler, K. S. (2007). Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Res Hum Genet; 10:423–433.
- Betjemann, R. S., Johnson, E. P., Barnard, H., Boada, R., Filley, C. M., Filipek, P. A., … & Pennington, B. F. (2010). Genetic Covariation Between Brain Volumes and IQ, Reading Performance, and Processing Speed. Behavior genetics, 40(2), 135-145.
- Bishop, E. G., Cherny, S. S., Corley, R., Plomin, R., DeFries, J. C., & Hewitt, J. K. (2003). Development genetic analysis of general cognitive ability from 1 to 12 years in a sample of adoptees, biological siblings, and twins. Intelligence, 31, 31-49.
- Bouchard Jr, T. J. (1983). Do Environmental Similarities Explain the Similarity in Intelligence of Identical Twins Reared Apart?. Intelligence, 7(2), 175-184.
- Bouchard, T. J. Jr., Lykken, D. T., McGue, M., Segal, N. L. & Tellegen, A. (1990). Sources of human psychological differences: The Minnesota Study of Twins Reared Apart. Science, 250, 223-228.
- Brant, A. M., Haberstick, B. C., Corley, R. P., Wadsworth, S. J., DeFries, J. C., & Hewitt, J. K. (2009). The Developmental Etiology of High IQ. Behavior genetics, 39(4), 393-405.
- Brant, A. M., Munakata, Y., Boomsma, D. I., DeFries, J. C., Haworth, C. M., Keller, M. C., … & Hewitt, J. K. (2013). The nature and nurture of high IQ: an extended sensitive period for intellectual development. Psychol. Sci. 24 1487–1495.
- Briley, D. A., & Tucker-Drob, E. M. (2013). Explaining the Increasing Heritability of Cognitive Ability Across Development: A Meta-Analysis of Longitudinal Twin and Adoption Studies. Psychological Science, 24, 1704–1713.
- Calvin, C. M., Deary, I. J., Webbink, D., Smith, P., Fernandes, C., Lee, S. H., … & Visscher, P. M. (2012). Multivariate Genetic Analyses of Cognition and Academic Achievement from Two Population Samples of 174,000 and 166,000 School Children. Behavior genetics, 42(5), 699-710.
- Chabris, C. F., Hebert, B. M., Benjamin, D. J., Beauchamp, J., Cesarini, D., van der Loos, M., et al. (2012). Most reported genetic associations with general intelligence are probably false positives. Psychological Science, 23, 1314–1323.
- Chipuer, H. M., Rovine, M. J., & Plomin, R. (1990). LISREL Modeling: Genetic and Environmental Influences on IQ Revisited. Intelligence, 14, 11−29.
- Chow, B. W. Y., Ho, C. S. H., Wong, S. W. L., Waye, M. M., & Bishop, D. V. (2013). Generalist genes and cognitive abilities in Chinese twins. Developmental science, 16(2), 260-268.
- Daniels, M., Devlin, B., & Roeder, K. (1997). Of Genes and IQ. In Devlin, B., Fienberg, S. E. Resnick, D. P., and Roeder, K., (eds.), Intelligence, Genes, and Success (pp. 45-70). Springer New York.
- Davies, G., Tenesa, A., Payton, A., Yang, J., Harris, S. E., Liewald, D., et al. (2011). Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Molecular Psychiatry, 16, 996–1005.
- Davis, O. S., Haworth, C. M., & Plomin, R. (2009a). Dramatic Increase in Heritability of Cognitive Development From Early to Middle Childhood: An 8-Year Longitudinal Study of 8,700 Pairs of Twins. Psychological Science, 20(10), 1301-1308.
- Davis, O. S. P., Haworth, C. M. A., & Plomin, R. (2009b). Learning abilities and disabilities: Generalist genes in early adolescence. Cognitive Neuropsychiatry, 14, 312–331.
- Deary, I. J., Spinath, F. M., & Bates, T. C. (2006). Genetics of intelligence. European Journal of Human Genetics., 14, 690−700.
- Deary, I. J., Yang, J., Davies, G., Harris, S. E., Tenesa, A., Liewald, D., … & Visscher, P. M. (2012). Genetic contributions to stability and change in intelligence from childhood to old age. Nature, 482(7384), 212-215.
- Deary, I. J., Penke, L., & Johnson, W. (2010). The neuroscience of human intelligence differences. Nature Rev. Neurosci. 11, 201–211.
- DeFries, J. C., Plomin, R., & LaBuda, M. C. (1987). Genetic Stability of Cognitive Development From Childhood to Adulthood. Developmental Psychology, 23, 4-12.
- Derks, E. M., Dolan, C. V., & Boomsma, D. I. (2006). A Test of the Equal Environment Assumption (EEA) in Multivariate Twin Studies. Twin Research and Human Genetics, 9(03), 403-411.
- Devlin, B., Daniels, M., & Roeder, K. (1997). The heritability of IQ. Nature, 388(6641), 468-471.
- Dickens, W. T., & Flynn, J. R. (2001). Heritability Estimates Versus Large Environmental Effects: The IQ Paradox Resolved. Psychological review, 108(2), 346.
- Docherty, S. J., Kovas, Y., Petrill, S. A., & Plomin, R. (2010). Generalist genes analysis of DNA markers associated with mathematical ability and disability reveals shared influence across ages and abilities. BMC genetics, 11(1), 61.
- Eichler E.E., Flint J., Gibson G., Kong A., Leal S.M., Moore J.H., Nadeau J.H. (2010). Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 11:446–450.
- Finkel, D., & Pedersen, N. L. (2001). Sources of environmental influence on cognitive abilities in adulthood. In E. L. Grigorenko, & R. J. Sternberg (Eds.), Family environment and intellectual functioning (pp. 173−194). Mahwah: Lawrence Erlbaum Associates.
- Fulker, D. W., DeFries, J. C., & Plomin, R. (1988). Genetic influence on general mental ability increases between infancy and middle childhood. Nature, 336, 767-769.
- Grandy, T. H., Werkle-Bergner, M., Chicherio, C., Lövdén, M., Schmiedek, F., & Lindenberger, U. (2013). Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage, 79, 10-18.
- Haworth, C. M., Dale, P. S., & Plomin, R. (2009a). Generalist Genes and High Cognitive Abilities. Behavior Genetics, 39, 437-445.
- Haworth, C. M., Wright, M. J., Martin, N. W., Martin, N. G., Boomsma, D. I., Bartels, M., … & Plomin, R. (2009b). A Twin Study of the Genetics of High Cognitive Ability Selected from 11,000 Twin Pairs in Six Studies from Four Countries. Behav Genet, 2009. 39(4): p. 359-70.
- Haworth, C. M., Wright, M. J., Luciano, M., Martin, N. G., de Geus, E. J., van Beijsterveldt, C. E., et al. (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 1−9.
- Hill, W. G., Goddard, M. E., & Visscher, P. M. (2008). Data and Theory Point to Mainly Additive Genetic Variance for Complex Traits. PLoS Genetics, 4, e1000008.
- Hoekstra, R. A., Bartels, M., & Boomsma, D. I. (2007). Longitudinal genetic study of verbal and nonverbal IQ from early childhood to young adulthood. Learning and Individual Differences, 17(2), 97-114.
- Horn, J. M., Loehlin, J. C., & Willerman, L. (1982). Aspects of the inheritance of intellectual abilities. Behavior Genetics, 12(5), 479-516.
- Pol, H. E. H., Schnack, H. G., Posthuma, D., Mandl, R. C., Baaré, W. F., van Oel, C., … & Kahn, R. S. (2006). Genetic Contributions to Human Brain Morphology and Intelligence. J. Neurosci. 26, 10235–10242.
- Jacobs N, van Gestel S, Derom C, Thiery E, Vernon P, Derom R, Vlietinck R. (2001). Heritability estimates of intelligence in twins: Effect of chorion type. Behav Genet 31:209-217.
- Jensen, A. R. (1970). IQ’s of Identical Twins Reared Apart. Behavior Genetics, 1, 133−148.
- Jensen, A. R. (1973). Educability and Group Differences. New York: Harper and Row.
- Jensen, A. R. (1976). The problem of genotype-environment correlation in the estimation of heritability from monozygotic and dizygotic twins. Acta geneticae medicae et gemellologiae, 25, 86.
- Jensen, A. R. (1980). Bias in Mental Testing. New York: Free Press.
- Jensen, A. R. 1998). The g Factor: The Science of Mental Ability. Westport, CT: Praeger.
- Jensen, A. R. (2006). Clocking the Mind: Mental Chronometry and Individual Differences. Elsevier.
- Johnson, W., Bouchard, T. J., McGue, M., Segal, N. L., Tellegen, A., Keyes, M., et al. (2007). Genetic and environmental influences on the Verbal-Perceptual-Image Rotation (VPR) model of the structure of mental abilities in the Minnesota study of twins reared apart. Intelligence, 35, 542–562.
- Jung, R. E., & Haier, R. J. (2007). The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences 30(2):135–87.
- Kovas, Y., & Plomin, R. (2006a). Generalist genes: implications for the cognitive sciences. Trends in Cognitive Sciences, 10, 198-203.
- Kovas, Y., & Plomin, R. (2006b). Response to Marcus and Rabagliati ‘Genes and domain specificity’. Trends in Cognitive Sciences, 10(9), 398.
- Lee, J. J. (2010). Review of intelligence and how to get it: Why schools and cultures count, RE Nisbett, Norton, New York, NY (2009). ISBN: 9780393065053.
- Loehlin, J. C. (1989). Partitioning Environmental and Genetic Contributions to Behavioral Development. American Psychologist, 44, 1285-1292.
- Loehlin, J. C., & DeFries, J. C. (1987). Genotype-Environment Correlation and IQ. Behavior Genetics, 17(3), 263-277.
- Loehlin, J. C., Horn, J. M., & Willerman, L. (1989). Modeling IQ change: Evidence from the Texas Adoption Project. Child development, 993-1004.
- Loehlin, J. C., & Horn, J. M. (2000). Stoolmiller on Restriction of Range in Adoption Studies: A Comment. Behavior genetics, 30(3), 245-247.
- Luciano, M., Posthuma, D., Wright, M. J., de Geus, E. J., Smith, G. A., Geffen, G. M., … & Martin, N. G. (2005). Perceptual speed does not cause intelligence, and intelligence does not cause perceptual speed. Biological Psychology, 70(1), 1-8.
- Luciano, M., Evans, D.M., Hansell, N.K., Medland, S.E., Montgomery, G.W., Martin, N.G., Wright, M.J. & Bates, T.C. (2013). A genome-wide association study for reading and language abilities in two population cohorts. Genes Brain Behav 12, 645–652.
- Luders, E., Narr, K. L., Thompson, P. M., & Toga, A. W. (2009). Neuroanatomical correlates of intelligence. Intelligence, 37(2), 156-163.
- Lyons, M. J., York, T. P., Franz, C. E., Grant, M. D., Eaves, L. J., Jacobson, K. C., … & Kremen, W. S. (2009). Genes Determine Stability and the Environment Determines Change in Cognitive Ability During 35 Years of Adulthood. Psychological science, 20(9), 1146-1152.
- Maher B. (2008). The case of the missing heritability. Nature 456: 18–21.
- Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A. et al. (2009). Finding the missing heritability for complex diseases. Nat Rev Genet 461:747–753.
- McCall, R. B. (1981). Nature-Nurture and the Two Realms of Development: A Proposed Integration with Respect to Mental Development. Child Development, 1-12.
- McCartney, K., Harris, M., & Bernieri, F. (1990). Growing up and growing apart: A developmental meta-analysis of twin studies. Psychological Bulletin, 107, 226-237.
- McGue, M., Keyes, M., Sharma, A., Elkins, I., Legrand, L., Johnson, W., & Iacono, W. G. (2007). The Environments of Adopted and Non-adopted Youth: Evidence on Range Restriction From the Sibling Interaction and Behavior Study (SIBS). Behavior Genetics, 37(3), 449-462.
- Molenaar D., van der Sluis S., Boomsma D.I., Haworth C.M., Hewitt J.K., Martin N.G. et al. (2013). Genotype by environment interactions in cognitive ability: a survey of 14 studies from four countries covering four age groups. Behav Genet 43:208–219.
- Neubauer, A. C., & Fink, A. (2009). Intelligence and neural efficiency. Neuroscience & Biobehavioral Reviews, 33(7), 1004-1023.
- Panizzon, M. S., Vuoksimaa, E., Spoon, K. M., Jacobson, K. C., Lyons, M. J., Franz, C. E., … & Kremen, W. S. (2014). Genetic and environmental influences on general cognitive ability: Is g a valid latent construct?. Intelligence, 43, 65-76.
- Penke, L., Maniega, S. M., Murray, C., Gow, A. J., Hernández, M. C. V., Clayden, J. D., … & Deary, I. J. (2010). A General Factor of Brain White Matter Integrity Predicts Information Processing Speed in Healthy Older People. J Neurosci; 30: 7569–7574.
- Penke, L., Maniega, S. M., Bastin, M. E., Hernandez, M. V., Murray, C., Royle, N. A., … & Deary, I. J. (2012). Brain white matter tract integrity as a neural foundation for general intelligence. Molecular psychiatry, 17(10), 1026-1030.
- Peper, J. S., Brouwer, R. M., Boomsma, D. I., Kahn, R. S., Pol, H., & Hilleke, E. (2007). Genetic Influences on Human Brain Structure: A Review of Brain Imaging Studies in Twins. Hum. Brain Mapp. 28, 464–473.
- Petrill, S. A., & Deater-Deckard, K. (2004). The heritability of general cognitive ability: A within-family adoption design. Intelligence, 32(4), 403-409.
- Plomin, R., & Loehlin, J. C. (1989). Direct and Indirect IQ Heritability Estimates: A Puzzle. Behavior Genetics, 19(3), 331-342.
- Plomin, R., DeFries, J. C., & Fulker, D. W. ([1988] 2006). Nature and Nurture during Infancy and Early Childhood. Cambridge University Press.
- Plomin, R., DeFries, J. C., Knopik, V. S., & Neiderhiser, J. M. (2012). Behavioral genetics (6th ed.). New York: Worth Publishers.
- Plomin, R., Fulker, D. W., Corley, R., & DeFries, J. C. (1997). Nature, Nurture, and Cognitive Development from 1 to 16 Years: A Parent-Offspring Adoption Study. Psychological Science, 8, 442−447.
- Plomin, R., & Kovas, Y. (2005). Generalist Genes and Learning Disabilities. Psychological Bulletin, 131, 592–617.
- Plomin, R., Kovas, Y., & Haworth, C. (2007). Generalist Genes: Genetic Links Between Brain, Mind, and Education. Mind, Brain, and Education, 1(1), 11-19.
- Plomin, R., Haworth, C. M., Meaburn, E. L., Price, T. S., & Davis, O. S. (2013). Common DNA markers can account for more than half of the genetic influence on cognitive abilities. Psychological Science, 24, 562–568.
- Posthuma, D., de Geus, E. J., & Boomsma, D. I. (2003). Genetic Contributions to Anatomical, Behavioral, and Neurophysiological Indices of Cognition. In R. Plomin, J. C. Defries, I. W. Craig, & P. McGuffin (Eds.), Behavioral Genetics in the Postgenomic Era (pp. 141–161). Washington, DC: American Psychological Association.
- Price, A. L., Patterson, N. J., Plenge, R. M., Weinblatt, M. E., Shadick, N. A., & Reich, D. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genet. 38, 904–909.
- Price, A.L., Zaitlen, N.A., Reich, D. & Patterson, N. (2010). New approaches to population stratification in genome-wide association studies. Nat. Rev. Genet. 11, 459–463.
- Rice, T., Fulker, D. W., & DeFries, J. C. (1986). Multivariate Path Analysis of Specific Cognitive Abilities in the Colorado Adoption Project. Behav. Genet. 16:107-125.
- Rice, T., Fulker, D. W., DeFries, J. C., & Plomin, R. (1988). Path Analysis of IQ during Infancy and Early Childhood and an Index of the Home Environment in the Colorado Adoption Project. Colorado Adoption Project. Intelligence, 12(1), 27-45.
- Rice, T., Carey, G., Fulker, D. W., & DeFries, J. C. (1989). Multivariate Path Analysis of Specific Cognitive Abilities in the Colorado Adoption Project: Conditional Path Model of Assortative Mating. Behavior Genetics, 19, 195−207.
- Rowe, D. C. (1994). The Limits of Family Influence: Genes, Experience, and Behavior. New York: Guilford Press.
- Rowe, D. C. (2003). Assessing Genotype-Environment Interactions and Correlations in the Postgenomic Era. R. In Plomin, J. C. DeFries, I. W. Craig, P. McGuffin, (Eds.), Behavioral Genetics in the Postgenomic Era (pp. 71–86). Washington, D.C.: American Psychological Association.
- Rushton, J. P., & Ankney, C. D. (2009). Whole Brain Size and General Mental Ability: A Review. International Journal of Neuroscience, 119(5), 692-732.
- Scarr, S., & Carter-Saltzman, L. (1979). Twin Method: Defense of a Critical Assumption. Behavior Genetics, 9(6), 527-542.
- Scarr, S., & McCartney, K. (1983). How people make their own environments: A theory of genotype‑environment effects. Child Development, 54, 424‑435.
- Scarr, S., & Weinberg, R. A. (1978). The Influence of ‘Family Background’ on Intellectual Attainment. American Sociological Review, 674-692.
- Schmitt, J. E., Wallace, G. L., Rosenthal, M. A., Molloy, E. A., Ordaz, S., Lenroot, R., … & Giedd, J. N. (2007a). A multivariate analysis of neuroanatomic relationships in a genetically informative pediatric sample. Neuroimage 35, 70–82.
- Schmitt, J. E., Eyler, L. T., Giedd, J. N., Kremen, W. S., Kendler, K. S., & Neale, M. C. (2007b). Review of Twin and Family Studies on Neuroanatomic Phenotypes and Typical Neurodevelopment. Twin Res. Hum. Genet. 10, 683–694.
- Segal, N. L. (1997). Same-Age Unrelated Siblings: A Unique Test of Within-Family Environmental Influences on IQ Similarity. Journal of Educational Psychology, 89(2), 381.
- Segal, N. L. (2000). Virtual Twins: New Findings on Within-Family Environmental Influences on Intelligence. Journal of Educational Psychology, 92(3), 442.
- Segal, N. L., & Hershberger, S. L. (2005). Virtual twins and intelligence: Updated and new analyses of within-family environmental influences. Personality and Individual Differences, 39(6), 1061-1073.
- Segal, N. L., McGuire, S. A., Havlena, J., Gill, P., & Hershberger, S. L. (2007). Intellectual similarity of virtual twin pairs: Developmental trends. Personality and individual differences, 42(7), 1209-1219.
- Segal, N. L., McGuire, S. A., & Stohs, J. H. (2012). What virtual twins reveal about general intelligence and other behaviors. Personality and individual differences, 53(4), 405-410.
- Sesardic, N. (2005). Making Sense of Heritability. Cambridge University Press.
- Shikishima, C., Hiraishi, K., Yamagata, S., Sugimoto, Y., Takemura, R., Ozaki, K., … & Ando, J. (2009). Is g an entity? A Japanese twin study using syllogisms and intelligence tests. Intelligence, 37(3), 256-267.
- Smit, D. J., Luciano, M., Bartels, M., Van Beijsterveldt, C. E., Wright, M. J., Hansell, N. K., … & Boomsma, D. I. (2010). Heritability of Head Size in Dutch and Australian Twin Families at Ages 0–50 Years. Twin Research and Human Genetics, 13(04), 370-380.
- Stoolmiller, M. (1998). Correcting Estimates of Shared Environmental Variance for Range Restriction in Adoption Studies Using a Truncated Multivariate Normal Model. Behavior Genetics, 28(6), 429-441.
- Stoolmiller, M. (1999). Implications of the Restricted Range of Family Environments for Estimates of Heritability and Nonshared Environment in Behavior-Genetic Adoption Studies. Psychological bulletin, 125(4), 392.
- Taal, H. R., St Pourcain, B., Thiering, E., Das, S., Mook-Kanamori, D. O., Warrington, N. M., … & Vernooij, M. W. (2012). Common variants at 12q15 and 12q24 are associated with infant head circumference. Nat. Genet. 44, 532–538.
- Thompson, L. A., Plomin, R., & DeFries, J. C. (1985). Parent-Infant Resemblance for General and Specific Cognitive Abilities in the Colorado Adoption Project. Intelligence, 9(1), 1-13.
- Trzaskowski, M., Yang, J., Visscher, P. M., & Plomin, R. (2013a). DNA evidence for strong genetic stability and increasing heritability of intelligence from age 7 to 12. Molecular psychiatry, 19(3), 380-384.
- Trzaskowski, M., Davis, O. P., DeFries, J., Yang, J., Visscher, P., & Plomin, R. (2013b). DNA Evidence for Strong Genome-Wide Pleiotropy of Cognitive and Learning Abilities. Behavior genetics, 43(4), 267-273.
- Trzaskowski, M., Dale, P. S., & Plomin, R. (2013c). No Genetic Influence for Childhood Behavior Problems From DNA Analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 52(10), 1048-1056.
- Trzaskowski, M., Shakeshaft, N. G., & Plomin, R. (2013d). Intelligence indexes generalist genes for cognitive abilities. Intelligence, 41(5), 560-565.
- Van Der Maas, H. L., Dolan, C. V., Grasman, R. P., Wicherts, J. M., Huizenga, H. M., & Raijmakers, M. E. (2006). A Dynamical Model of General Intelligence: The Positive Manifold of Intelligence by Mutualism. Psychological review, 113(4), 842.
- van Leeuwen, M., van den Berg, S.M., Boomsma, D.I. (2008). A twin-family study of general IQ. Learning and Individual Differences 18, 76–88.
- van Leeuwen, M., Peper, J. S., van den Berg, S. M., Brouwer, R. M., Hulshoff Pol, H. E., Kahn, R. S., & Boomsma, D. I. (2009). A genetic analysis of brain volumes and IQ in children. Intelligence, 37(2), 181-191.
- van Soelen, I.L.C., Brouwer, R.M., van Leeuwen, M., Kahn, R.S., Hulshoff Pol, H.E., Boomsma, D.I. (2011). Heritability of Verbal and Performance Intelligence in a Pediatric Longitudinal Sample. Twin Res. Hum. Genet. 14, 119–128.
- van Soelen, I. L. C., Brouwer, R. M., van Baal, G. C. M., Schnack, H. G., Peper, J. S., Collins, D. L., … & Hulshoff Pol, H. E. (2012). Genetic influences on thinning of the cerebral cortex during development. Neuroimage, 59(4), 3871-3880.
- Visscher, P.M., Medland, S.E., Ferreira, M.A., Morley, K.I., Zhu, G., Cornes, B.K., Montgomery, G.W., and Martin, N.G. (2006). Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet. 2, e41.
- Visscher, P. M., Yang, J., & Goddard, M. E. (2010). A Commentary on ‘Common SNPs Explain a Large Proportion of the Heritability for Human Height’ by Yang et al. (2010). Twin Research and Human Genetics, 13(06), 517-524.
- Visscher, P. M., Brown, M. A., McCarthy, M. I., & Yang, J. (2012). Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24.
- Vuoksimaa, E., Panizzon, M. S., Chen, C. H., Fiecas, M., Eyler, L. T., Fennema-Notestine, C., … & Kremen, W. S. (2014). The Genetic Association Between Neocortical Volume and General Cognitive Ability Is Driven by Global Surface Area Rather Than Thickness. Cerebral Cortex, bhu018.
- Wadsworth, S. J., Corley, R. P., Hewitt, J. K., Plomin, R., & DeFries, J. C. (2002). Parent-offspring resemblance for reading performance at 7, 12 and 16 years of age in the Colorado Adoption Project. Journal of Child Psychology and Psychiatry, 43(6), 769-774.
- Yang, J. A., Lee, S. H., Goddard, M. E., & Visscher, P. M. (2011a). GCTA: A tool for genome-wide complex trait analysis. The American Journal of Human Genetics, 88, 76–82.
- Yang, J., Manolio, T.A., Pasquale, L.R., Boerwinkle, E., Caporaso, N., Cunningham, J.M., de Andrade, M., Feenstra, B., Feingold, E., Hayes, M.G., et al. (2011b). Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 43, 519–525.
- Zaitlen N., Kraft P. (2012). Heritability in the genome-wide association era. Hum Genet 131: 1655-1664.
- Zuk, O., Hechter, E., Sunyaev, S. R., & Lander, E. S. (2012). The mystery of missing heritability: genetic interactions create phantom heritability. Proc. Natl Acad. Sci. USA 109, 1193–1198.